当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting the Mg/TMSCl/Dipolar Solvent System for Dearomatic Silylation of Aryl Carbonyl Compounds: Substrate Scope, Transformations, and Mechanistic Studies
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.joc.2c01178 Nan-Nan Li 1 , Meng Li 1 , Jia-Ni Gao 1 , Zhong Zhang 1 , Jian-Bo Xie 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.joc.2c01178 Nan-Nan Li 1 , Meng Li 1 , Jia-Ni Gao 1 , Zhong Zhang 1 , Jian-Bo Xie 1, 2
Affiliation
|
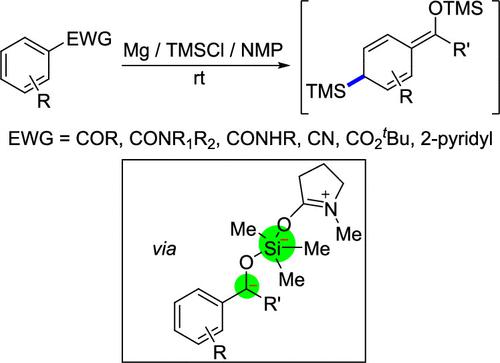
Dearomatic silylation of arene derivatives is an intriguing synthetic target, which represents an elegant extension of Birch reduction and produces silylated cyclohexene derivatives with great potential of further transformation. Herein, we report a systematic study on dearomatic silylation of aryl carbonyl compounds with Mg and the TMSCl/NMP adduct. The protocol displays a wide range of substrate scope, including alkyl aryl ketones, aromatic amides, benzonitriles, tert-butyl benzoates, and even 2,2′-bipyridines. Synthetic utility is demonstrated using the products as versatile substrate in various transformations. The detailed mechanism is presented with both control experimental analyses and theoretical calculations. An unusual five-coordinated silicon dianion intermediate is first proposed and described here. The selectivity is influenced by the relative rates of single electron reductions (the TMSCl/NMP adduct versus the substrate) and the steric effects.
中文翻译:

重新审视用于芳基羰基化合物脱芳基硅烷化的 Mg/TMSCl/偶极溶剂系统:底物范围、转化和机理研究
芳烃衍生物的脱芳基甲硅烷基化是一个有趣的合成目标,它代表了 Birch 还原的优雅延伸,并产生具有进一步转化潜力的甲硅烷基化环己烯衍生物。在此,我们报告了一项关于芳基羰基化合物与 Mg 和 TMSCl/NMP 加合物脱芳基甲硅烷基化的系统研究。该协议显示了广泛的底物范围,包括烷基芳基酮、芳香酰胺、苯甲腈、叔-苯甲酸丁酯,甚至 2,2'-联吡啶。使用这些产品作为各种转化中的多功能底物,证明了合成效用。通过控制实验分析和理论计算提出了详细的机制。这里首先提出并描述了一种不寻常的五配位硅二阴离子中间体。选择性受单电子还原的相对速率(TMSCl/NMP 加合物与底物)和空间效应的影响。
更新日期:2022-07-29
中文翻译:

重新审视用于芳基羰基化合物脱芳基硅烷化的 Mg/TMSCl/偶极溶剂系统:底物范围、转化和机理研究
芳烃衍生物的脱芳基甲硅烷基化是一个有趣的合成目标,它代表了 Birch 还原的优雅延伸,并产生具有进一步转化潜力的甲硅烷基化环己烯衍生物。在此,我们报告了一项关于芳基羰基化合物与 Mg 和 TMSCl/NMP 加合物脱芳基甲硅烷基化的系统研究。该协议显示了广泛的底物范围,包括烷基芳基酮、芳香酰胺、苯甲腈、叔-苯甲酸丁酯,甚至 2,2'-联吡啶。使用这些产品作为各种转化中的多功能底物,证明了合成效用。通过控制实验分析和理论计算提出了详细的机制。这里首先提出并描述了一种不寻常的五配位硅二阴离子中间体。选择性受单电子还原的相对速率(TMSCl/NMP 加合物与底物)和空间效应的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号