当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Oxidative Amination of α-Olefins with Indoles
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.orglett.2c02190 Shreeja Bhatt 1 , Ya-Nong Wang 1 , Hoang T P Pham 1 , Kami L Hull 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.orglett.2c02190 Shreeja Bhatt 1 , Ya-Nong Wang 1 , Hoang T P Pham 1 , Kami L Hull 1
Affiliation
|
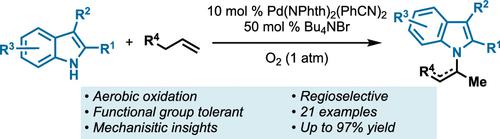
Herein we report the use of indoles, one of the most common nitrogen-containing heterocycles in FDA-approved drugs, as nucleophiles in the Pd-catalyzed aza-Wacker reaction. This N-functionalization of indoles is a Markovnikov selective olefin functionalization of simple alkenes using catalytic Pd(NPhth)2(PhCN)2 and O2 as the terminal oxidant in the presence of catalytic Bu4NBr. Various substituted indoles and alkenes are found to participate; 21 examples are presented with yields ranging from 41 to 97% isolated yield. Additionally, lactams and oxazolidinones are shown to participate under the reaction conditions. Mechanistic investigations suggest that the phthalimide ligand and Bu4NBr additive slow undesired side reactions: indole decomposition and olefin isomerization, respectively.
中文翻译:

钯催化的 α-烯烃与吲哚的氧化胺化反应
在这里,我们报告了吲哚的用途,吲哚是 FDA 批准的药物中最常见的含氮杂环化合物之一,作为 Pd 催化的氮杂瓦克反应中的亲核试剂。这种吲哚的N官能化是在催化 Bu 4 NBr存在下使用催化 Pd(NPhth) 2 (PhCN) 2和 O 2作为末端氧化剂的简单烯烃的马尔可夫尼科夫选择性烯烃官能化。发现各种取代的吲哚和烯烃参与其中;提供了 21 个示例,其产率范围为 41% 至 97% 的分离产率。此外,内酰胺和恶唑烷酮显示参与反应条件。机理研究表明邻苯二甲酰亚胺配体和 Bu 4NBr 添加剂可减缓不需要的副反应:分别为吲哚分解和烯烃异构化。
更新日期:2022-07-29
中文翻译:

钯催化的 α-烯烃与吲哚的氧化胺化反应
在这里,我们报告了吲哚的用途,吲哚是 FDA 批准的药物中最常见的含氮杂环化合物之一,作为 Pd 催化的氮杂瓦克反应中的亲核试剂。这种吲哚的N官能化是在催化 Bu 4 NBr存在下使用催化 Pd(NPhth) 2 (PhCN) 2和 O 2作为末端氧化剂的简单烯烃的马尔可夫尼科夫选择性烯烃官能化。发现各种取代的吲哚和烯烃参与其中;提供了 21 个示例,其产率范围为 41% 至 97% 的分离产率。此外,内酰胺和恶唑烷酮显示参与反应条件。机理研究表明邻苯二甲酰亚胺配体和 Bu 4NBr 添加剂可减缓不需要的副反应:分别为吲哚分解和烯烃异构化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号