Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2022-07-29 , DOI: 10.1016/j.psep.2022.07.060 Haojie Zhang , Chan Zhou , Hanxuan Zeng , Zhou Shi , Huiying Wu , Lin Deng
|
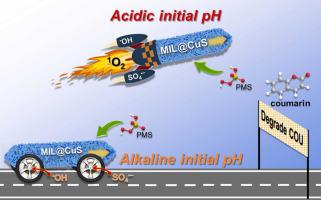
A novel rod-shaped MIL-88A(Fe)@CuS featured with sulfur vacancies (SV) were constructed as heterogeneous catalysts for activating peroxymonosulfate (PMS) for coumarin (COU) degradation. A series of xMIL@CuS were obtained according to the mass content of MIL-88A(Fe) in the composites (x, wt% = 50 %, 65 %, and 80 %). Among them, 65 % MIL@CuS hold the best performance, and realized a complete COU removal (30 μM) in 7 min (0.2 g/L 65 %MIL@CuS and 0.5 mM PMS). The degradation was much more favorable in acidic initial pH than alkaline initial pH. The calculated reaction rate constants at initial pH of 3.0, 5.0, 6.0 and 9.0 were 0.903, 0.729, 0.650 and 0.095 min−1, respectively. Electron paramagnetic resonance (EPR) analysis, radical scavenging tests and mechanism exploration indicated that the main difference in degradation under acidic and alkaline pH came from the yield of 1O2. In initial pH = 3.0 condition, SV and lattice oxygen on 65 %MIL@CuS participated in the generation of 1O2, greatly increasing the content of 1O2 (11.6 × 10−11 M) and promoting the degradation. While under initial alkaline condition (pH = 9.0), 1O2 were basically produced from the reaction between Cu(II) and PMS, resulting in a low yield (1.7 × 10−11 M) and lower degradation. Besides, 65 %MIL@CuS maintained excellent reusability with low metal ions leaching, and the degradation exceeded 98.0 % even in the fifth run. Overall, this work provided an efficient and stable activator for activating PMS to degrade refractory organics, and managed to disclose the activation mechanisms under acidic and alkaline pH.
中文翻译:

新型硫空位采用 MIL-88A(Fe)@CuS 棒活化过氧单硫酸盐降解香豆素:酸性和碱性 pH 值下不同的活性氧生成途径
构建了一种具有硫空位 (S V )的新型棒状 MIL-88A(Fe)@CuS作为非均相催化剂,用于活化过氧单硫酸盐 (PMS ) 降解香豆素 (COU)。根据复合材料中MIL-88A(Fe)的质量含量( x , wt% = 50 %, 65 %, and 80 %)得到一系列x MIL@CuS。其中,65 % MIL@CuS 的性能最好,并在 7 分钟内实现了完全的 COU 去除(30 μM)(0.2 g/L 65 %MIL@CuS 和 0.5 mM PMS)。酸性初始 pH 值的降解比碱性初始 pH 值更有利。在初始 pH 值 3.0、5.0、6.0 和 9.0 时计算的反应速率常数为 0.903、0.729、0.650 和 0.095 min -1, 分别。电子顺磁共振(EPR)分析、自由基清除试验和机理探索表明,酸性和碱性pH下降解的主要区别在于1 O 2的产率。在初始pH = 3.0条件下,65%MIL@CuS上的S V和晶格氧参与了1 O 2的生成,大大增加了1 O 2 (11.6 × 10 -11 M)的含量,促进了降解。而在初始碱性条件下(pH = 9.0),1 O 2主要由 Cu(II) 和 PMS 之间的反应产生,导致产率低(1.7 × 10 -11M) 和更低的降解。此外,65 %MIL@CuS 保持了良好的可重复使用性,金属离子浸出率低,即使在第五次运行中降解率也超过了 98.0 %。总体而言,这项工作为激活 PMS 以降解难降解有机物提供了一种高效且稳定的活化剂,并设法揭示了酸性和碱性 pH 值下的活化机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号