Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synergistic Integration and Pharmacomechanical Function of Enzyme-Magnetite Nanoparticle Swarms for Low-Dose Fast Thrombolysis
Small ( IF 13.3 ) Pub Date : 2022-07-29 , DOI: 10.1002/smll.202202848 Xiuzhen Tang 1, 2, 3 , Laliphat Manamanchaiyaporn 2, 3, 4 , Qi Zhou 5 , Chenyang Huang 4 , Lihuang Li 6 , Ziqiao Li 2 , Longchen Wang 1 , Jienan Wang 1 , Lei Ren 6 , Tiantian Xu 3 , Xiaohui Yan 2 , Yuanyi Zheng 1
Small ( IF 13.3 ) Pub Date : 2022-07-29 , DOI: 10.1002/smll.202202848 Xiuzhen Tang 1, 2, 3 , Laliphat Manamanchaiyaporn 2, 3, 4 , Qi Zhou 5 , Chenyang Huang 4 , Lihuang Li 6 , Ziqiao Li 2 , Longchen Wang 1 , Jienan Wang 1 , Lei Ren 6 , Tiantian Xu 3 , Xiaohui Yan 2 , Yuanyi Zheng 1
Affiliation
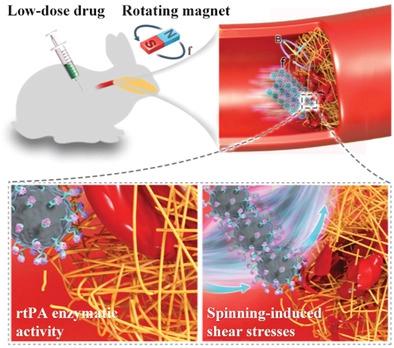
|
Magnetic micro-/nanoparticles are extensively explored over the past decade as active diagnostic/therapeutic agents for minimally invasive medicine. However, sufficient function integration on these miniaturized bodies toward practical applications remains challenging. This work proposes a synergistic strategy via integrating particle functionalization and bioinspired swarming, demonstrated by recombinant tissue plasminogen activator modified magnetite nanoparticles (rtPA-Fe3O4 NPs) for fast thrombolysis in vivo with low drug dosage. The synthesized rtPA-Fe3O4 NPs exhibit superior magnetic performance, high biocompatibility, and thrombolytic enzyme activity. Benefiting from a customized magnetic operation system designed for animal experiments and preclinical development, these agglomeration-free NPs can assemble into micro-/milli-scale swarms capable of robust maneuver and reconfigurable transformation for on-demand tasks in complex biofluids. Specifically, the spinning mode of the swarm exerts focused fluid shear stresses while rubbing on the thrombus surface, constituting a mechanical force for clot breakdown. The synergy of the NPs’ inherent enzymatic effect and swarming-triggered fluid forces enables amplified efficacy of thrombolysis in an in vivo occlusion model of rabbit carotid artery, using lower drug concentration than clinical dosage. Furthermore, swarming-enhanced ultrasound signals aid in imaging-guided treatment. Therefore, the pharmacomechanical NP swarms herein represent an injectable thrombolytic tool joining advantages of intravenous drug therapy and robotic intervention.
中文翻译:

酶-磁铁矿纳米粒子群在低剂量快速溶栓中的协同整合和药理作用
在过去的十年中,磁性微/纳米颗粒作为微创医学的活性诊断/治疗剂得到了广泛的探索。然而,在这些小型化身体上对实际应用进行充分的功能集成仍然具有挑战性。这项工作提出了一种通过整合粒子功能化和仿生蜂群的协同策略,通过重组组织纤溶酶原激活剂修饰的磁铁矿纳米粒子(rtPA-Fe 3 O 4 NPs)在体内以低药物剂量进行快速溶栓证明。合成的rtPA-Fe 3 O 4NPs表现出优异的磁性能、高生物相容性和溶栓酶活性。受益于为动物实验和临床前开发设计的定制磁操作系统,这些无团聚的纳米粒子可以组装成微/毫尺度的群体,能够为复杂生物流体中的按需任务提供稳健的机动和可重构转换。具体来说,群体的旋转模式在血栓表面摩擦的同时施加集中的流体剪切应力,构成血栓破裂的机械力。NPs 固有的酶促效应和蜂群触发的流体力的协同作用使得在兔颈动脉的体内闭塞模型中使用比临床剂量更低的药物浓度来放大溶栓的功效。此外,蜂群增强的超声信号有助于成像引导治疗。因此,本文的药物力学 NP 群代表了一种可注射溶栓工具,结合了静脉药物治疗和机器人干预的优势。
更新日期:2022-07-29
中文翻译:

酶-磁铁矿纳米粒子群在低剂量快速溶栓中的协同整合和药理作用
在过去的十年中,磁性微/纳米颗粒作为微创医学的活性诊断/治疗剂得到了广泛的探索。然而,在这些小型化身体上对实际应用进行充分的功能集成仍然具有挑战性。这项工作提出了一种通过整合粒子功能化和仿生蜂群的协同策略,通过重组组织纤溶酶原激活剂修饰的磁铁矿纳米粒子(rtPA-Fe 3 O 4 NPs)在体内以低药物剂量进行快速溶栓证明。合成的rtPA-Fe 3 O 4NPs表现出优异的磁性能、高生物相容性和溶栓酶活性。受益于为动物实验和临床前开发设计的定制磁操作系统,这些无团聚的纳米粒子可以组装成微/毫尺度的群体,能够为复杂生物流体中的按需任务提供稳健的机动和可重构转换。具体来说,群体的旋转模式在血栓表面摩擦的同时施加集中的流体剪切应力,构成血栓破裂的机械力。NPs 固有的酶促效应和蜂群触发的流体力的协同作用使得在兔颈动脉的体内闭塞模型中使用比临床剂量更低的药物浓度来放大溶栓的功效。此外,蜂群增强的超声信号有助于成像引导治疗。因此,本文的药物力学 NP 群代表了一种可注射溶栓工具,结合了静脉药物治疗和机器人干预的优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号