Water Research ( IF 11.4 ) Pub Date : 2022-07-29 , DOI: 10.1016/j.watres.2022.118919 Yanqing Cong 1 , Lidong Shen 1 , Baimei Wang 1 , Jianlai Cao 1 , Zixuan Pan 1 , Ziyu Wang 1 , Kai Wang 1 , Qiangbiao Li 1 , Xuchun Li 1
|
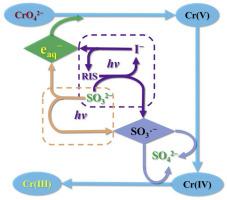
Efficient removal of toxic hexavalent chromium (Cr(VI)) under alkaline conditions is still a challenge due to the relatively low reactivity of CrO42−. This study proposed a new sulfite/iodide/UV process to remove Cr(VI). The removal of Cr(VI) followed pseudo-zero-order kinetics at alkaline pHs, and was enhanced by sulfite and iodide with synergy. Compared with sulfite/UV, iodide in sulfite/iodide/UV showed about 40 times higher concentration-normalized enhancement for Cr(VI) removal, and reduced the requirement of sulfite ([S(IV)]0/[Cr(VI)]0 of about 2.1:1) by more than 90%. The Cr(VI) removal was accelerated by decreasing pH and by increasing temperature, and was slightly influenced by dissolved oxygen, carbonate, and humic acid. The process was still effective in real surface water and industrial wastewater. Mechanism and pathways of Cr(VI) removal were revealed by quenching experiments, competition kinetic analysis, product identification and quantification, and mass and electron balance. Both eaq− and SO3•− were responsible for Cr(VI) removal, making contributions of about 75% and 25%, respectively. When eaq− mainly reacted with Cr(VI), SO3•− participated in reduction of Cr(V) and Cr(IV) intermediates, with Cr(III), S2O62−, and SO42− as the final products. A model was developed to predict removal kinetics of Cr(VI), and well interpreted the roles of S(IV) and iodide in the process. This study sheds light on mechanism of Cr(VI) removal at alkaline pHs by kinetic modeling, and thus advances the applicability of this promising process for water decontamination.
中文翻译:

亚硫酸盐/碘化物/UV 在碱性 pH 条件下有效去除 Cr(VI):机理和建模
由于 CrO 4 2-的反应性相对较低,在碱性条件下有效去除有毒的六价铬 (Cr(VI)) 仍然是一个挑战。本研究提出了一种新的亚硫酸盐/碘化物/UV 工艺去除 Cr(VI)。在碱性 pH 条件下,Cr(VI) 的去除遵循准零级动力学,并通过亚硫酸盐和碘化物的协同作用增强。与亚硫酸盐/UV相比,亚硫酸盐/碘化物/UV中的碘化物对Cr(VI)的去除显示出约40倍的浓度归一化增强,并降低了亚硫酸盐([S(IV)] 0 /[Cr(VI)] 0约 2.1:1) 超过 90%。降低 pH 值和升高温度会加速 Cr(VI) 的去除,但受溶解氧、碳酸盐和腐殖酸的影响较小。该工艺在实际地表水和工业废水中仍然有效。通过淬火实验、竞争动力学分析、产物鉴定和定量以及质量和电子平衡揭示了 Cr(VI) 去除的机理和途径。e aq -和 SO 3 •-都对 Cr(VI) 的去除负责,贡献率分别约为 75% 和 25%。当e aq -主要与Cr(VI)反应时,SO 3 •-参与了 Cr(V) 和 Cr(IV) 中间体的还原,最终产品为Cr(III)、S 2 O 6 2-和 SO 4 2- 。开发了一个模型来预测 Cr(VI) 的去除动力学,并很好地解释了 S(IV) 和碘化物在该过程中的作用。本研究通过动力学建模阐明了在碱性 pH 条件下去除 Cr(VI) 的机制,从而提高了这种有前途的水净化工艺的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号