当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting of overall water splitting activity by regulating the electron distribution over the active sites of Ce doped NiCo–LDH and atomic level understanding of the catalyst by DFT study
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-07-29 , DOI: 10.1039/d2ta04647d Hariharan N. Dhandapani 1, 2 , D. Mahendiran 1, 3 , Arun Karmakar 1, 2 , Pandiarajan Devi 1, 3 , Sreenivasan Nagappan 1, 2 , Ragunath Madhu 1, 2 , Krishnendu Bera 1, 2 , Palanichamy Murugan 1, 3 , B. Ramesh Babu 1, 2 , Subrata Kundu 1, 2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-07-29 , DOI: 10.1039/d2ta04647d Hariharan N. Dhandapani 1, 2 , D. Mahendiran 1, 3 , Arun Karmakar 1, 2 , Pandiarajan Devi 1, 3 , Sreenivasan Nagappan 1, 2 , Ragunath Madhu 1, 2 , Krishnendu Bera 1, 2 , Palanichamy Murugan 1, 3 , B. Ramesh Babu 1, 2 , Subrata Kundu 1, 2
Affiliation
|
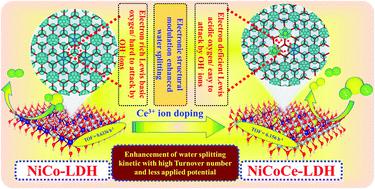
Electrolysis of water plays a vital role in the generation of hydrogen as compared to the other methods such as hydrolysis of metal hydrides, steam reforming, coal gasification, and oxidation of methane gas. All those methods have several disadvantages, and hence, the development of efficient electrocatalysts with low cost and long stability towards water electrolysis for solving the energy crisis is needed. An LDH material is an efficient candidate for the OER process but not as efficient for the HER process. Here, in this study, the doping of Ce3+ over NiCo–LDH/NF modulates the electronic structure of the active metal sites, enhancing the performance of both OER and HER processes and affecting the microstructure, electrocatalytic performance, and electronic structure of NiCo–LDH/NF. In OER and HER, the catalyst Ce@NiCo–LDH demanding overpotential values of 250 mV and 134 mV to attain a current density of 50 mA cm−2 and Tafel slope values of 98 and 98.6 mV dec−1, respectively. Furthermore, the overall water splitting was carried out by a chronoamperometric study for Ce-doped NiCo–LDH/NF showing electrochemical performance for 36 h with the current density at 10 mA cm−2 at 1.68 V vs. RHE. A DFT study at the atomic level of pristine and Ce doped NiCo–LDH revealed that the doping of Ce improves the electronic conductivity of the catalyst, and Co-3d states dominate the water splitting activity as compared to other Ni-3d and Ce-4f states. This work provides an alternate route to design a highly efficient and cost effective catalyst for global clean energy production.
中文翻译:

通过调节 Ce 掺杂的 NiCo-LDH 活性位点上的电子分布和通过 DFT 研究对催化剂的原子水平理解来提高整体水分解活性
与金属氢化物的水解、蒸汽重整、煤气化和甲烷气体的氧化等其他方法相比,水的电解在氢气的产生中起着至关重要的作用。所有这些方法都有几个缺点,因此,需要开发成本低、对水电解具有长稳定性的高效电催化剂来解决能源危机。LDH 材料是 OER 工艺的有效候选材料,但对于 HER 工艺效率不高。这里,在本研究中,Ce 3+的掺杂过 NiCo-LDH/NF 调节活性金属位点的电子结构,提高 OER 和 HER 过程的性能,并影响 NiCo-LDH/NF 的微观结构、电催化性能和电子结构。在 OER 和 HER 中,催化剂 Ce@NiCo-LDH 需要 250 mV 和 134 mV 的过电位值才能分别获得 50 mA cm -2的电流密度和 98 和 98.6 mV dec -1的 Tafel 斜率值。此外,通过计时电流分析对 Ce 掺杂的 NiCo-LDH/NF 进行了整体水分解,显示了 36 小时的电化学性能,电流密度为 10 mA cm -2,电压为 1.68 V vs.RHE。在原始和 Ce 掺杂的 NiCo-LDH 的原子水平上的 DFT 研究表明,Ce 的掺杂提高了催化剂的电子电导率,与其他 Ni-3d 和 Ce-4f 相比,Co-3d 态主导水分解活性状态。这项工作为设计用于全球清洁能源生产的高效且具有成本效益的催化剂提供了另一种途径。
更新日期:2022-07-29
中文翻译:

通过调节 Ce 掺杂的 NiCo-LDH 活性位点上的电子分布和通过 DFT 研究对催化剂的原子水平理解来提高整体水分解活性
与金属氢化物的水解、蒸汽重整、煤气化和甲烷气体的氧化等其他方法相比,水的电解在氢气的产生中起着至关重要的作用。所有这些方法都有几个缺点,因此,需要开发成本低、对水电解具有长稳定性的高效电催化剂来解决能源危机。LDH 材料是 OER 工艺的有效候选材料,但对于 HER 工艺效率不高。这里,在本研究中,Ce 3+的掺杂过 NiCo-LDH/NF 调节活性金属位点的电子结构,提高 OER 和 HER 过程的性能,并影响 NiCo-LDH/NF 的微观结构、电催化性能和电子结构。在 OER 和 HER 中,催化剂 Ce@NiCo-LDH 需要 250 mV 和 134 mV 的过电位值才能分别获得 50 mA cm -2的电流密度和 98 和 98.6 mV dec -1的 Tafel 斜率值。此外,通过计时电流分析对 Ce 掺杂的 NiCo-LDH/NF 进行了整体水分解,显示了 36 小时的电化学性能,电流密度为 10 mA cm -2,电压为 1.68 V vs.RHE。在原始和 Ce 掺杂的 NiCo-LDH 的原子水平上的 DFT 研究表明,Ce 的掺杂提高了催化剂的电子电导率,与其他 Ni-3d 和 Ce-4f 相比,Co-3d 态主导水分解活性状态。这项工作为设计用于全球清洁能源生产的高效且具有成本效益的催化剂提供了另一种途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号