当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Umpolung (3+2) Cycloadditions of Iminoesters with α,β-Unsaturated-2-acyl Imidazoles for the Synthesis of Substituted Pyrrolidines
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.orglett.2c01438 Mamta Gill 1 , Arko Das 1 , Vinod K Singh 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acs.orglett.2c01438 Mamta Gill 1 , Arko Das 1 , Vinod K Singh 1
Affiliation
|
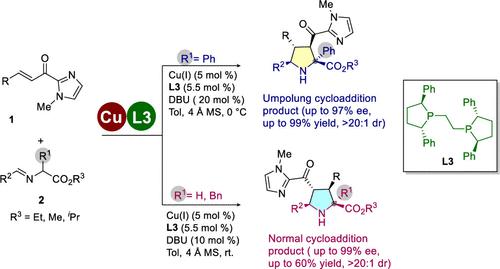
An enantioselective (3+2) cycloaddition reaction of iminoesters occurring with opposite regioselectivity is reported. This reaction provides chiral polysubstituted pyrrolidines with high enantioselectivities (up to 97%), diastereoselectivities (>20:1), and yields (up to 99%). Interestingly, changing the alpha-substituents of the iminoesters from an aryl to an aliphatic (benzyl) group or hydrogen resulted in the formation of normal (3+2) cycloaddition products, also in excellent enantioselectivities.
中文翻译:

亚胺酯与 α,β-不饱和-2-酰基咪唑的不对称 Umpolung (3+2) 环加成用于合成取代吡咯烷
报道了以相反的区域选择性发生的亚氨基酯的对映选择性 (3+2) 环加成反应。该反应提供了具有高对映选择性(高达 97%)、非对映选择性(>20:1)和产率(高达 99%)的手性多取代吡咯烷。有趣的是,将亚氨基酯的 α-取代基从芳基变为脂肪族(苄基)基团或氢会导致形成正常的 (3+2) 环加成产物,同时具有出色的对映选择性。
更新日期:2022-07-29
中文翻译:

亚胺酯与 α,β-不饱和-2-酰基咪唑的不对称 Umpolung (3+2) 环加成用于合成取代吡咯烷
报道了以相反的区域选择性发生的亚氨基酯的对映选择性 (3+2) 环加成反应。该反应提供了具有高对映选择性(高达 97%)、非对映选择性(>20:1)和产率(高达 99%)的手性多取代吡咯烷。有趣的是,将亚氨基酯的 α-取代基从芳基变为脂肪族(苄基)基团或氢会导致形成正常的 (3+2) 环加成产物,同时具有出色的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号