当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Access to α- and β-N-Glycosylamine Derivatives by a Metal Free O-to-N [3,3]-Sigmatropic Rearrangement
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-07-27 , DOI: 10.1002/ejoc.202200804 Debora Pratesi 1 , Stefania Mirabella 2 , Giulia Petrucci 2 , Camilla Matassini 2 , Cristina Faggi 3 , Francesca Cardona 1 , Andrea Goti 4
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-07-27 , DOI: 10.1002/ejoc.202200804 Debora Pratesi 1 , Stefania Mirabella 2 , Giulia Petrucci 2 , Camilla Matassini 2 , Cristina Faggi 3 , Francesca Cardona 1 , Andrea Goti 4
Affiliation
|
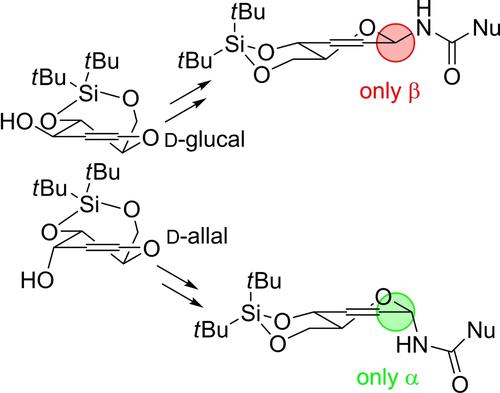
Glycals substituted at C-3 with a carbamate group undergo, upon dehydration, a prompt allyl cyanate to isocyanate rearrangement to the isomeric N-glycosyl isocyanates, which give N-glycosyl carbamates and ureas by one-pot addition of alcohols or amines. The rearrangement occurs with complete stereoselectivity via a [3,3]-sigmatropic mechanism, affording α- or β-linked amines depending on the configuration at C-3 of the carbohydrate.
中文翻译:

通过无金属 O-to-N [3,3]-Sigmatropic 重排获得 α- 和 β-N-糖基胺衍生物的立体特异性
在 C-3 处被氨基甲酸酯基团取代的糖醇在脱水后会迅速发生氰酸烯丙酯到异氰酸酯重排,形成异构的N-糖基异氰酸酯,通过一锅法添加醇或胺得到N-糖基氨基甲酸酯和脲。重排通过 [3,3]-sigmatropic 机制以完全立体选择性发生,根据碳水化合物 C-3 的构型提供 α- 或 β- 连接的胺。
更新日期:2022-07-27
中文翻译:

通过无金属 O-to-N [3,3]-Sigmatropic 重排获得 α- 和 β-N-糖基胺衍生物的立体特异性
在 C-3 处被氨基甲酸酯基团取代的糖醇在脱水后会迅速发生氰酸烯丙酯到异氰酸酯重排,形成异构的N-糖基异氰酸酯,通过一锅法添加醇或胺得到N-糖基氨基甲酸酯和脲。重排通过 [3,3]-sigmatropic 机制以完全立体选择性发生,根据碳水化合物 C-3 的构型提供 α- 或 β- 连接的胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号