当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Native SAD phasing at room temperature
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-07-27 , DOI: 10.1107/s2059798322006799 Jack B Greisman 1 , Kevin M Dalton 1 , Candice J Sheehan 1 , Margaret A Klureza 2 , Igor Kurinov 3 , Doeke R Hekstra 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2022-07-27 , DOI: 10.1107/s2059798322006799 Jack B Greisman 1 , Kevin M Dalton 1 , Candice J Sheehan 1 , Margaret A Klureza 2 , Igor Kurinov 3 , Doeke R Hekstra 1
Affiliation
|
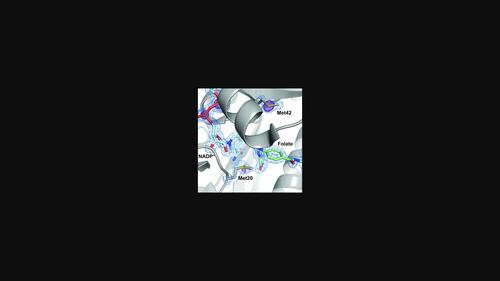
Single-wavelength anomalous diffraction (SAD) is a routine method for overcoming the phase problem when solving macromolecular structures. This technique requires the accurate measurement of intensities to determine differences between Bijvoet pairs. Although SAD experiments are commonly conducted at cryogenic temperatures to mitigate the effects of radiation damage, such temperatures can alter the conformational ensemble of the protein and may impede the merging of data from multiple crystals due to non-uniform freezing. Here, a strategy is presented to obtain high-quality data from room-temperature, single-crystal experiments. To illustrate the strengths of this approach, native SAD phasing at 6.55 keV was used to solve four structures of three model systems at 295 K. The resulting data sets allow automatic phasing and model building, and reveal alternate conformations that reflect the structure of proteins at room temperature.
中文翻译:

室温下的原生 SAD 定相
单波长反常衍射(SAD)是解决大分子结构时克服相位问题的常规方法。该技术需要精确测量强度以确定 Bijvoet 对之间的差异。尽管 SAD 实验通常在低温下进行,以减轻辐射损伤的影响,但这种温度可能会改变蛋白质的构象整体,并且可能由于不均匀冷冻而阻碍多个晶体数据的合并。在这里,提出了一种从室温单晶实验中获取高质量数据的策略。为了说明这种方法的优势,使用 6.55 keV 的天然 SAD 定相来求解 295 K 下三个模型系统的四种结构。生成的数据集允许自动定相和模型构建,并揭示反映蛋白质结构的替代构象室温。
更新日期:2022-07-27
中文翻译:

室温下的原生 SAD 定相
单波长反常衍射(SAD)是解决大分子结构时克服相位问题的常规方法。该技术需要精确测量强度以确定 Bijvoet 对之间的差异。尽管 SAD 实验通常在低温下进行,以减轻辐射损伤的影响,但这种温度可能会改变蛋白质的构象整体,并且可能由于不均匀冷冻而阻碍多个晶体数据的合并。在这里,提出了一种从室温单晶实验中获取高质量数据的策略。为了说明这种方法的优势,使用 6.55 keV 的天然 SAD 定相来求解 295 K 下三个模型系统的四种结构。生成的数据集允许自动定相和模型构建,并揭示反映蛋白质结构的替代构象室温。











































 京公网安备 11010802027423号
京公网安备 11010802027423号