当前位置:
X-MOL 学术
›
J. Magnes. Alloys
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ formation of multiple catalysts for enhancing the hydrogen storage of MgH2 by adding porous Ni3ZnC0.7/Ni loaded carbon nanotubes microspheres
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2022-07-27 , DOI: 10.1016/j.jma.2022.07.004 Bing Zhang , Xiubo Xie , Yukun Wang , Chuanxin Hou , Xueqin Sun , Yuping Zhang , Xiaoyang Yang , Ronghai Yu , Wei Du
Journal of Magnesium and Alloys ( IF 15.8 ) Pub Date : 2022-07-27 , DOI: 10.1016/j.jma.2022.07.004 Bing Zhang , Xiubo Xie , Yukun Wang , Chuanxin Hou , Xueqin Sun , Yuping Zhang , Xiaoyang Yang , Ronghai Yu , Wei Du
|
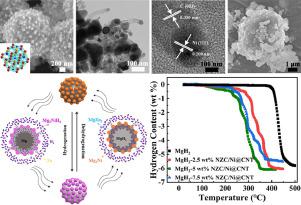
MgH is considered one of the most promising hydrogen storage materials because of its safety, high efficiency, high hydrogen storage quantity and low cost characteristics. But some shortcomings are still existed: high operating temperature and poor hydrogen absorption dynamics, which limit its application. Porous NiZnC/Ni loaded carbon nanotubes microspheres (NZC/Ni@CNT) is prepared by facile filtration and calcination method. Then the different amount of NZC/Ni@CNT (2.5, 5.0 and 7.5 wt%) is added to the MgH by ball milling. Among the three samples with different amount of NZC/Ni@CNT (2.5, 5.0 and 7.5 wt%), the MgH-5 wt% NZC/Ni@CNT composite exhibits the best hydrogen storage performances. After testing, the MgH-5 wt% NZC/Ni@CNT begins to release hydrogen at around 110 °C and hydrogen absorption capacity reaches 2.34 wt% H at 80 °C within 60 min. Moreover, the composite can release about 5.36 wt% H at 300 °C. In addition, hydrogen absorption and desorption activation energies of the MgH-5 wt% NZC/Ni@CNT composite are reduced to 37.28 and 84.22 KJ/mol H, respectively. The generated MgNiH/MgNi can serve as a "hydrogen pump" that plays the main role in providing more activation sites and hydrogen diffusion channels which promotes H dissociation during hydrogen absorption process. In addition, the evenly dispersed Zn and MgZn in Mg and MgH could provide sites for Mg/MgH nucleation and hydrogen diffusion channel. This attempt clearly proved that the bimetallic carbide NiZnC is a effective additive for the hydrogen storage performances modification of MgH, and the facile synthesis of the NiZnC/Ni@CNT can provide directions of better designing high performance carbide catalysts for improving MgH.
中文翻译:

添加多孔Ni3ZnC0.7/Ni负载碳纳米管微球原位形成多种催化剂增强MgH2储氢
MgH因其安全、高效、储氢量高、成本低等特点被认为是最有前途的储氢材料之一。但仍存在工作温度高、吸氢动力学差等缺点,限制了其应用。采用简便的过滤和煅烧方法制备了多孔NiZnC/Ni负载碳纳米管微球(NZC/Ni@CNT)。然后通过球磨将不同量的 NZC/Ni@CNT(2.5、5.0 和 7.5 wt%)添加到 MgH 中。在不同NZC/Ni@CNT含量(2.5、5.0和7.5 wt%)的三个样品中,MgH-5 wt% NZC/Ni@CNT复合材料表现出最好的储氢性能。经测试,MgH-5 wt% NZC/Ni@CNT在110℃左右开始放氢,在80℃下60 min内吸氢能力达到2.34 wt% H2。此外,该复合材料在300℃下可以释放约5.36wt%的H。此外,MgH-5 wt% NZC/Ni@CNT复合材料的吸氢和解吸活化能分别降低至37.28和84.22 KJ/mol H。生成的MgNiH/MgNi可以作为“氢泵”,其主要作用是提供更多的活化位点和氢扩散通道,促进吸氢过程中H的解离。此外,Mg和MgH中均匀分散的Zn和MgZn可以为Mg/MgH成核和氢扩散通道提供位点。这一尝试清楚地证明了双金属碳化物NiZnC是一种有效的MgH储氢性能改性添加剂,并且NiZnC/Ni@CNT的简便合成可以为更好地设计用于改善MgH的高性能碳化物催化剂提供方向。
更新日期:2022-07-27
中文翻译:

添加多孔Ni3ZnC0.7/Ni负载碳纳米管微球原位形成多种催化剂增强MgH2储氢
MgH因其安全、高效、储氢量高、成本低等特点被认为是最有前途的储氢材料之一。但仍存在工作温度高、吸氢动力学差等缺点,限制了其应用。采用简便的过滤和煅烧方法制备了多孔NiZnC/Ni负载碳纳米管微球(NZC/Ni@CNT)。然后通过球磨将不同量的 NZC/Ni@CNT(2.5、5.0 和 7.5 wt%)添加到 MgH 中。在不同NZC/Ni@CNT含量(2.5、5.0和7.5 wt%)的三个样品中,MgH-5 wt% NZC/Ni@CNT复合材料表现出最好的储氢性能。经测试,MgH-5 wt% NZC/Ni@CNT在110℃左右开始放氢,在80℃下60 min内吸氢能力达到2.34 wt% H2。此外,该复合材料在300℃下可以释放约5.36wt%的H。此外,MgH-5 wt% NZC/Ni@CNT复合材料的吸氢和解吸活化能分别降低至37.28和84.22 KJ/mol H。生成的MgNiH/MgNi可以作为“氢泵”,其主要作用是提供更多的活化位点和氢扩散通道,促进吸氢过程中H的解离。此外,Mg和MgH中均匀分散的Zn和MgZn可以为Mg/MgH成核和氢扩散通道提供位点。这一尝试清楚地证明了双金属碳化物NiZnC是一种有效的MgH储氢性能改性添加剂,并且NiZnC/Ni@CNT的简便合成可以为更好地设计用于改善MgH的高性能碳化物催化剂提供方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号