Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Artificial Palmitoylation of Proteins Controls the Lipid Domain-Selective Anchoring on Biomembranes and the Raft-Dependent Cellular Internalization
Langmuir ( IF 3.7 ) Pub Date : 2022-07-26 , DOI: 10.1021/acs.langmuir.2c01205 Kazuki Uchida 1 , Hiroki Obayashi 1 , Kosuke Minamihata 1 , Rie Wakabayashi 1 , Masahiro Goto 1, 2 , Naofumi Shimokawa 3 , Masahiro Takagi 3 , Noriho Kamiya 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2022-07-26 , DOI: 10.1021/acs.langmuir.2c01205 Kazuki Uchida 1 , Hiroki Obayashi 1 , Kosuke Minamihata 1 , Rie Wakabayashi 1 , Masahiro Goto 1, 2 , Naofumi Shimokawa 3 , Masahiro Takagi 3 , Noriho Kamiya 1, 2
Affiliation
|
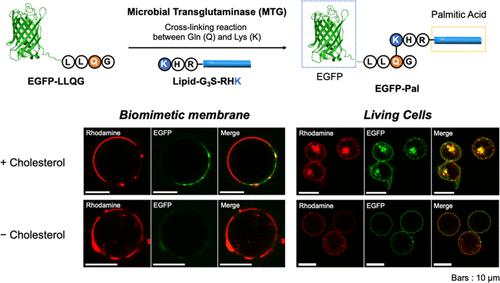
Protein palmitoylation, a post-translational modification, is universally observed in eukaryotic cells. The localization of palmitoylated proteins to highly dynamic, sphingolipid- and cholesterol-rich microdomains (called lipid rafts) on the plasma membrane has been shown to play an important role in signal transduction in cells. However, this complex biological system is not yet completely understood. Here, we used a combined approach where an artificial lipidated protein was applied to biomimetic model membranes and plasma membranes in cells to illuminate chemical and physiological properties of the rafts. Using cell-sized giant unilamellar vesicles, we demonstrated the selective partitioning of enhanced green fluorescent protein modified with a C-terminal palmitoyl moiety (EGFP-Pal) into the liquid-ordered phase consisting of saturated phospholipids and cholesterol. Using Jurkat T cells treated with an immunostimulant (concanavalin A), we observed the vesicular transport of EGFP-Pal. Further cellular studies with the treatment of methyl β-cyclodextrin revealed the cholesterol-dependent internalization of EGFP-Pal, which can be explained by a raft-dependent, caveolae-mediated endocytic pathway. The present synthetic approach using artificial and natural membrane systems can be further extended to explore the potential utility of artificially lipidated proteins at biological and artificial interfaces.
中文翻译:

蛋白质的人工棕榈酰化控制生物膜上的脂质结构域选择性锚定和依赖筏的细胞内化
蛋白质棕榈酰化是一种翻译后修饰,在真核细胞中普遍存在。棕榈酰化蛋白定位于质膜上高度动态的、富含鞘脂和胆固醇的微结构域(称为脂筏)已被证明在细胞中的信号转导中起重要作用。然而,这个复杂的生物系统还没有被完全理解。在这里,我们使用了一种组合方法,将人工脂化蛋白应用于仿生模型膜和细胞中的质膜,以阐明筏的化学和生理特性。使用细胞大小的巨大单层囊泡,我们展示了用 C 末端棕榈酰部分 (EGFP-Pal) 修饰的增强型绿色荧光蛋白选择性分配到由饱和磷脂和胆固醇组成的液体有序相中。使用用免疫刺激剂 (伴刀豆球蛋白 A) 处理的 Jurkat T 细胞,我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。
更新日期:2022-07-26
中文翻译:

蛋白质的人工棕榈酰化控制生物膜上的脂质结构域选择性锚定和依赖筏的细胞内化
蛋白质棕榈酰化是一种翻译后修饰,在真核细胞中普遍存在。棕榈酰化蛋白定位于质膜上高度动态的、富含鞘脂和胆固醇的微结构域(称为脂筏)已被证明在细胞中的信号转导中起重要作用。然而,这个复杂的生物系统还没有被完全理解。在这里,我们使用了一种组合方法,将人工脂化蛋白应用于仿生模型膜和细胞中的质膜,以阐明筏的化学和生理特性。使用细胞大小的巨大单层囊泡,我们展示了用 C 末端棕榈酰部分 (EGFP-Pal) 修饰的增强型绿色荧光蛋白选择性分配到由饱和磷脂和胆固醇组成的液体有序相中。使用用免疫刺激剂 (伴刀豆球蛋白 A) 处理的 Jurkat T 细胞,我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。我们观察到 EGFP-Pal 的囊泡运输。用甲基 β-环糊精处理的进一步细胞研究揭示了 EGFP-Pal 的胆固醇依赖性内化,这可以通过筏依赖性、小窝介导的内吞途径来解释。目前使用人工和天然膜系统的合成方法可以进一步扩展,以探索人工脂化蛋白质在生物和人工界面上的潜在用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号