Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porphyrin-Based Conjugated Microporous Polymers for Highly Efficient Adsorption of Metal Ions
Langmuir ( IF 3.7 ) Pub Date : 2022-07-25 , DOI: 10.1021/acs.langmuir.2c00681 Qi-Meige Hasi 1 , Zhi-Chao Han 1 , Yu-Ping Guo 1 , Jia-Le Yu 1 , Chao-Hu Xiao 2 , Yu-Han Zhang 1 , Li-Hua Chen 1
Langmuir ( IF 3.7 ) Pub Date : 2022-07-25 , DOI: 10.1021/acs.langmuir.2c00681 Qi-Meige Hasi 1 , Zhi-Chao Han 1 , Yu-Ping Guo 1 , Jia-Le Yu 1 , Chao-Hu Xiao 2 , Yu-Han Zhang 1 , Li-Hua Chen 1
Affiliation
|
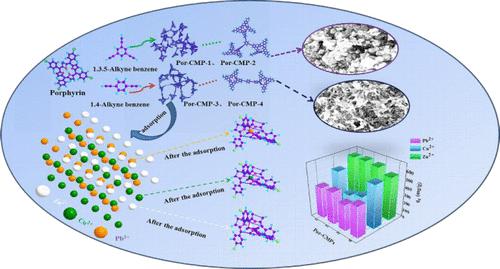
The capture and elimination of anions and cations from water have attracted a great deal of attention and are quite vital for clean production and environmental remediation. In this work, we present the synthesis of four porphyrin (Por)-based conjugated microporous polymers (CMPs, namely, Por-CMP-1–4), which were produced through a Sonogashira–Hagihara linked response using porphyrin and acetylene aromatic compounds as building blocks and used as absorbents to eliminate metal ions from water. The as-synthesized Por-CMP-1–4 exhibit an amorphous porous structure and outstanding caloric and physicochemical properties. Taking advantage of their larger specific surface areas, i.e., 541.47, 614.58, 382.38, and 677.90 m2 g–1 for Por-CMP-1–4, respectively, and their chelating active site that originated from the porphyrin ring, Por-CMP-1–4 show better Zn2+, Cu2+, and Pb2+ adsorption ability. Among them, Por-CMP-3 has the greatest adsorbability of 640 mg g–1 for Zn2+, with an adsorption efficiency of 80%, whereas its adsorption capacities for Cu2+ and Pb2+ ions were both 334 mg g–1, with an adsorption efficiency of 42% for Cu2+ and Pb2+. Employing Por-CMP-3 as a representative example, its adsorption kinetics has been systematically investigated. The adsorption behavior of Por-CMP-3 with respect to the Zn2+ ion is shown to exhibit pseudo-first-order kinetics and Langmuir isotherm modes. Meanwhile, the adsorption mechanism is discussed in detail, and it was thought it might be chelation, in which the nitrogen atoms with a single pair of electrons on the porphyrin ring interacted with metal ions to form stable chelation coordination bonds, thus removing metal ions selectively and effectively. Furthermore, Por-CMP-3 exhibited good reusability, retaining 60% of its Zn2+ removal rate after four continuous adsorptions.
中文翻译:

卟啉基共轭微孔聚合物高效吸附金属离子
水中阴离子和阳离子的捕获和消除引起了人们的广泛关注,对于清洁生产和环境修复至关重要。在这项工作中,我们提出了四种基于卟啉 (Por) 的共轭微孔聚合物 (CMP,即 Por-CMP-1-4) 的合成,它们是通过使用卟啉和乙炔芳族化合物的 Sonogashira-Hagihara 关联响应产生的积木和用作吸收剂以消除水中的金属离子。合成后的 Por-CMP-1-4 表现出无定形多孔结构和出色的热量和物理化学性质。利用它们更大的比表面积,即 541.47、614.58、382.38 和 677.90 m 2 g –1对于 Por-CMP-1-4 及其来源于卟啉环的螯合活性位点,Por-CMP-1-4 表现出更好的 Zn 2+、Cu 2+和 Pb 2+吸附能力。其中,Por-CMP-3对Zn 2+的吸附量最大,为640 mg g -1,吸附效率为80%,而对Cu 2+和Pb 2+离子的吸附量均为334 mg g - 1,对Cu 2+和Pb 2+的吸附效率为42%. 以 Por-CMP-3 为代表,对其吸附动力学进行了系统研究。Por-CMP-3 对Zn 2+离子的吸附行为表现出准一级动力学和Langmuir 等温线模式。同时对吸附机理进行了详细讨论,认为可能是螯合,卟啉环上具有单对电子的氮原子与金属离子相互作用形成稳定的螯合配位键,从而选择性地去除金属离子。并且有效。此外,Por-CMP-3 表现出良好的可重复使用性,连续四次吸附后仍保持其 60% 的 Zn 2+去除率。
更新日期:2022-07-25
中文翻译:

卟啉基共轭微孔聚合物高效吸附金属离子
水中阴离子和阳离子的捕获和消除引起了人们的广泛关注,对于清洁生产和环境修复至关重要。在这项工作中,我们提出了四种基于卟啉 (Por) 的共轭微孔聚合物 (CMP,即 Por-CMP-1-4) 的合成,它们是通过使用卟啉和乙炔芳族化合物的 Sonogashira-Hagihara 关联响应产生的积木和用作吸收剂以消除水中的金属离子。合成后的 Por-CMP-1-4 表现出无定形多孔结构和出色的热量和物理化学性质。利用它们更大的比表面积,即 541.47、614.58、382.38 和 677.90 m 2 g –1对于 Por-CMP-1-4 及其来源于卟啉环的螯合活性位点,Por-CMP-1-4 表现出更好的 Zn 2+、Cu 2+和 Pb 2+吸附能力。其中,Por-CMP-3对Zn 2+的吸附量最大,为640 mg g -1,吸附效率为80%,而对Cu 2+和Pb 2+离子的吸附量均为334 mg g - 1,对Cu 2+和Pb 2+的吸附效率为42%. 以 Por-CMP-3 为代表,对其吸附动力学进行了系统研究。Por-CMP-3 对Zn 2+离子的吸附行为表现出准一级动力学和Langmuir 等温线模式。同时对吸附机理进行了详细讨论,认为可能是螯合,卟啉环上具有单对电子的氮原子与金属离子相互作用形成稳定的螯合配位键,从而选择性地去除金属离子。并且有效。此外,Por-CMP-3 表现出良好的可重复使用性,连续四次吸附后仍保持其 60% 的 Zn 2+去除率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号