Water Research ( IF 11.4 ) Pub Date : 2022-07-25 , DOI: 10.1016/j.watres.2022.118896 Ning Li 1 , Yanshan Wang 2 , Xiaoshuang Cheng 2 , Haoxi Dai 2 , Beibei Yan 2 , Guanyi Chen 3 , Li'an Hou 2 , Shaobin Wang 4
|
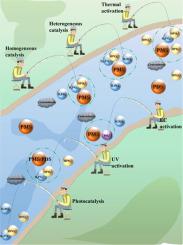
Currently, various strategies have been applied to activate persulfate (PS) for contaminant removal from water. However, the background phosphate ions in water affect PS activation and organic degradation, and the mechanism of their influence on the processes is still controversial. In this review, the possible effects of different phosphate forms (HPO42−, H2PO4−, and PO43−) on PS activation and contaminant degradation were systematically evaluated and summarized. Specifically, HPO42− promotes contaminant degradation in direct peroxymonosulfate (PMS) oxidation and thermal/PMS systems, while it exhibits inhibition to thermal/peroxodisulfate (PDS) and ultraviolet (UV)/PDS systems. Meanwhile, H2PO4− inhibits most oxidation processes based on PMS and PDS, except for non-metal dominated and metal assisted PMS systems. Coexisting HPO42− and H2PO4− could present beneficial effects in thermal, Co2+ and non-metal activated and metal assisted PMS systems. Nevertheless, their inhibitory effects were found in direct PMS oxidation, UV/PMS (or PDS) and metal dominated PMS systems. Generally, phosphate ions inhibit PMS/PDS activation through competing adsorption with PMS or PDS on the solid surface, forming a complex with metal ions, as well as occupying active sites on solid catalysts. In addition, phosphate ions can quench radicals for reduced degradation of contaminants. However, phosphate ions could weaken the bond dissociation energy via combining with PMS and contaminants or form a complex with Co2+, thus displaying a facilitative effect. This review further discusses major challenges and opportunities of PS activation with co-existing phosphates and will provide guidance for better PS utilization in real water treatment practice.
中文翻译:

磷酸根离子对水处理中过硫酸盐活化和有机物降解的影响及机制:综述
目前,已应用各种策略来激活过硫酸盐 (PS) 以去除水中的污染物。然而,水中的背景磷酸根离子影响PS活化和有机物降解,其影响过程的机制仍存在争议。在这篇综述中,系统地评估和总结了不同磷酸盐形式(HPO 4 2-、H 2 PO 4 -和 PO 4 3-)对 PS 活化和污染物降解的可能影响。具体来说,HPO 4 2−促进直接过一硫酸盐 (PMS) 氧化和热/PMS 系统中的污染物降解,同时对热/过二硫酸盐 (PDS) 和紫外线 (UV)/PDS 系统具有抑制作用。同时,H 2 PO 4 -抑制大多数基于 PMS 和 PDS 的氧化过程,除了非金属为主和金属辅助的 PMS 系统。共存的 HPO 4 2-和 H 2 PO 4 -可以在热、Co 2+和非金属活化和金属辅助 PMS 系统。然而,在直接 PMS 氧化、UV/PMS(或 PDS)和以金属为主的 PMS 系统中发现了它们的抑制作用。通常,磷酸根离子通过在固体表面与 PMS 或 PDS 竞争吸附,与金属离子形成络合物,以及占据固体催化剂上的活性位点来抑制 PMS/PDS 的活化。此外,磷酸根离子可以淬灭自由基以减少污染物的降解。然而,磷酸根离子会通过与 PMS 和污染物结合或与 Co 2+形成络合物而削弱键的解离能。,从而显示出促进作用。本综述进一步讨论了 PS 活化与共存磷酸盐的主要挑战和机遇,并将为在实际水处理实践中更好地利用 PS 提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号