Matrix Biology ( IF 4.5 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.matbio.2022.07.004 Kirstine S Nørregaard 1 , Henrik J Jürgensen 1 , Signe Z Ingvarsen 1 , Signe S Heltberg 1 , Christina E Hagensen 2 , Henrik Gårdsvoll 1 , Daniel H Madsen 3 , Ole N Jensen 2 , Lars H Engelholm 1 , Niels Behrendt 1
|
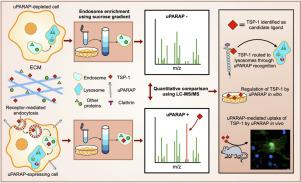
Thrombospondin-1 (TSP-1) is a matricellular protein with a multitude of functions in the pericellular and extracellular environment. We report a novel pathway for the regulation of extracellular TSP-1, governed by the endocytic collagen receptor, uPARAP (urokinase plasminogen activator receptor-associated protein; MRC2 gene product, also designated Endo180, CD280). First, using a novel proteomic approach for unbiased identification of ligands for endocytosis, we identify TSP-1 as a candidate ligand for specific uptake by uPARAP. We then show that uPARAP can efficiently internalize TSP-1 for lysosomal degradation, that this capability is not shared by other, closely related endocytic receptors and that uPARAP serves to regulate the extracellular levels of TSP-1 in vitro. Using wild type and uPARAP null mice, we also demonstrate uPARAP-mediated endocytosis of TSP-1 in dermal fibroblasts in vivo. Unlike other uPARAP ligands, the interaction with TSP-1 is sensitive to heparin and the responsible molecular motifs in uPARAP are overlapping, but not identical with those governing the interaction with collagens. Finally, we show that uPARAP can also mediate the endocytosis of TSP-2, a thrombospondin closely related to TSP-1, but not the more distantly related members of the same protein family, TSP-3, -4 and -5. These findings indicate that the role of uPARAP in ECM remodeling is not limited to the uptake of collagen for degradation but also includes an orchestrator function in the regulation of thrombospondins with numerous downstream effects. This is likely to be an important factor in the physiological and pathological roles of uPARAP in bone biology, fibrosis and cancer.
The proteomic data has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD031272.
中文翻译:

内吞受体 uPARAP 是细胞外血小板反应蛋白-1 的调节剂
Thrombospondin-1 (TSP-1) 是一种基质细胞蛋白,在细胞周和细胞外环境中具有多种功能。我们报告了一种调节细胞外 TSP-1 的新途径,该途径由内吞胶原蛋白受体 uPARAP(尿激酶纤溶酶原激活剂受体相关蛋白;MRC2 基因产物,也称为 Endo180、CD280)控制。首先,使用一种新颖的蛋白质组学方法来公正地鉴定内吞作用的配体,我们将 TSP-1 鉴定为 uPARAP 特异性摄取的候选配体。然后我们证明uPARAP可以有效地内化TSP-1以进行溶酶体降解,这种能力是其他密切相关的内吞受体所不具备的,并且uPARAP可以在体外调节TSP-1的细胞外水平。使用野生型和 uPARAP 无效小鼠,我们还证明了体内真皮成纤维细胞中 uPARAP 介导的 TSP-1 内吞作用。与其他 uPARAP 配体不同,与 TSP-1 的相互作用对肝素敏感,并且 uPARAP 中负责的分子基序重叠,但与控制与胶原蛋白相互作用的分子基序不同。最后,我们发现 uPARAP 还可以介导 TSP-2(一种与 TSP-1 密切相关的血小板反应蛋白)的内吞作用,但不能介导同一蛋白家族中关系较远的成员 TSP-3、-4 和 -5 的内吞作用。这些发现表明,uPARAP 在 ECM 重塑中的作用不仅限于摄取胶原蛋白进行降解,还包括调节血小板反应蛋白的协调器功能以及许多下游效应。这可能是 uPARAP 在骨生物学、纤维化和癌症中的生理和病理作用的重要因素。
蛋白质组数据已通过 PRIDE 合作伙伴存储库存入 ProteomeXchange 联盟,数据集标识符为 PXD031272。











































 京公网安备 11010802027423号
京公网安备 11010802027423号