当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Small-Molecule Inhibitors of Fibroblast Growth Factor 23 Signaling via In Silico Hot Spot Prediction and Molecular Docking to α-Klotho
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jcim.2c00633 Shih-Hsien Liu 1, 2 , Zhousheng Xiao 3 , Sambit K Mishra 4 , Julie C Mitchell 4 , Jeremy C Smith 1, 2 , L Darryl Quarles 3 , Loukas Petridis 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jcim.2c00633 Shih-Hsien Liu 1, 2 , Zhousheng Xiao 3 , Sambit K Mishra 4 , Julie C Mitchell 4 , Jeremy C Smith 1, 2 , L Darryl Quarles 3 , Loukas Petridis 1, 2
Affiliation
|
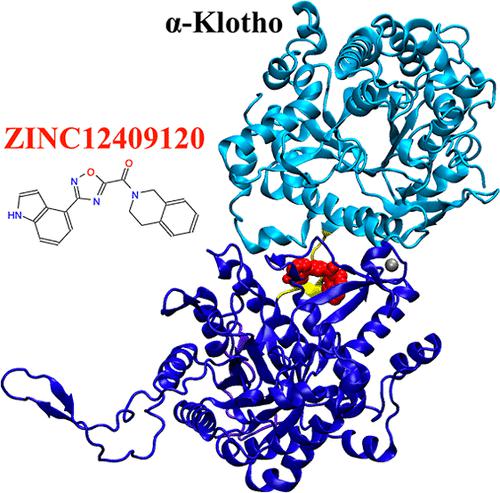
Fibroblast growth factor 23 (FGF23) is a therapeutic target for treating hereditary and acquired hypophosphatemic disorders, such as X-linked hypophosphatemic (XLH) rickets and tumor-induced osteomalacia (TIO), respectively. FGF23-induced hypophosphatemia is mediated by signaling through a ternary complex formed by FGF23, the FGF receptor (FGFR), and α-Klotho. Currently, disorders of excess FGF23 are treated with an FGF23-blocking antibody, burosumab. Small-molecule drugs that disrupt protein/protein interactions necessary for the ternary complex formation offer an alternative to disrupting FGF23 signaling. In this study, the FGF23:α-Klotho interface was targeted to identify small-molecule protein/protein interaction inhibitors since it was computationally predicted to have a large fraction of hot spots and two druggable residues on α-Klotho. We further identified Tyr433 on the KL1 domain of α-Klotho as a promising hot spot and α-Klotho as an appropriate drug-binding target at this interface. Subsequently, we performed in silico docking of ∼5.5 million compounds from the ZINC database to the interface region of α-Klotho from the ternary crystal structure. Following docking, 24 and 20 compounds were in the final list based on the lowest binding free energies to α-Klotho and the largest number of contacts with Tyr433, respectively. Five compounds were assessed experimentally by their FGF23-mediated extracellular signal-regulated kinase (ERK) activities in vitro, and two of these reduced activities significantly. Both these compounds were predicted to have favorable binding affinities to α-Klotho but not have a large number of contacts with the hot spot Tyr433. ZINC12409120 was found experimentally to disrupt FGF23:α-Klotho interaction to reduce FGF23-mediated ERK activities by 70% and have a half maximal inhibitory concentration (IC50) of 5.0 ± 0.23 μM. Molecular dynamics (MD) simulations of the ZINC12409120:α-Klotho complex starting from in silico docking poses reveal that the ligand exhibits contacts with residues on the KL1 domain, the KL1–KL2 linker, and the KL2 domain of α-Klotho simultaneously, thereby possibly disrupting the regular function of α-Klotho and impeding FGF23:α-Klotho interaction. ZINC12409120 is a candidate for lead optimization.
中文翻译:

通过计算机热点预测和 α-Klotho 分子对接鉴定成纤维细胞生长因子 23 信号传导的小分子抑制剂
成纤维细胞生长因子 23 (FGF23) 是治疗遗传性和获得性低磷血症疾病的治疗靶点,例如 X 连锁低磷血症 (XLH) 佝偻病和肿瘤诱发的骨软化症 (TIO)。 FGF23 诱导的低磷血症是通过 FGF23、FGF 受体 (FGFR) 和 α-Klotho 形成的三元复合物的信号传导介导的。目前,FGF23 过多疾病采用 FGF23 阻断抗体 burosumab 进行治疗。破坏三元复合物形成所需的蛋白质/蛋白质相互作用的小分子药物提供了破坏 FGF23 信号传导的替代方案。在本研究中,FGF23:α-Klotho 界面旨在识别小分子蛋白质/蛋白质相互作用抑制剂,因为计算预测其在 α-Klotho 上具有大部分热点和两个可药物残基。我们进一步确定 α-Klotho KL1 结构域上的 Tyr433 是一个有前途的热点,并且 α-Klotho 是该界面上合适的药物结合靶点。随后,我们将 ZINC 数据库中的约 550 万种化合物与三元晶体结构的 α-Klotho 界面区域进行了计算机对接。对接后,根据与 α-Klotho 的最低结合自由能和与 Tyr433 的接触次数最多,分别有 24 种和 20 种化合物进入最终列表。通过体外 FGF23 介导的细胞外信号调节激酶 (ERK) 活性对五种化合物进行了实验评估,其中两种化合物的活性显着降低。预计这两种化合物对 α-Klotho 具有良好的结合亲和力,但与热点 Tyr433 没有大量接触。 实验发现 ZINC12409120 可以破坏 FGF23:α-Klotho 相互作用,使 FGF23 介导的 ERK 活性降低 70%,半数抑制浓度 (IC 50 ) 为 5.0 ± 0.23 μM。从计算机对接姿势开始对 ZINC12409120:α-Klotho 复合物进行的分子动力学 (MD) 模拟表明,配体同时与 KL1 结构域、KL1-KL2 连接子和 α-Klotho 的 KL2 结构域上的残基接触,从而可能会破坏 α-Klotho 的正常功能并阻碍 FGF23:α-Klotho 相互作用。 ZINC12409120 是先导化合物优化的候选者。
更新日期:2022-07-22
中文翻译:

通过计算机热点预测和 α-Klotho 分子对接鉴定成纤维细胞生长因子 23 信号传导的小分子抑制剂
成纤维细胞生长因子 23 (FGF23) 是治疗遗传性和获得性低磷血症疾病的治疗靶点,例如 X 连锁低磷血症 (XLH) 佝偻病和肿瘤诱发的骨软化症 (TIO)。 FGF23 诱导的低磷血症是通过 FGF23、FGF 受体 (FGFR) 和 α-Klotho 形成的三元复合物的信号传导介导的。目前,FGF23 过多疾病采用 FGF23 阻断抗体 burosumab 进行治疗。破坏三元复合物形成所需的蛋白质/蛋白质相互作用的小分子药物提供了破坏 FGF23 信号传导的替代方案。在本研究中,FGF23:α-Klotho 界面旨在识别小分子蛋白质/蛋白质相互作用抑制剂,因为计算预测其在 α-Klotho 上具有大部分热点和两个可药物残基。我们进一步确定 α-Klotho KL1 结构域上的 Tyr433 是一个有前途的热点,并且 α-Klotho 是该界面上合适的药物结合靶点。随后,我们将 ZINC 数据库中的约 550 万种化合物与三元晶体结构的 α-Klotho 界面区域进行了计算机对接。对接后,根据与 α-Klotho 的最低结合自由能和与 Tyr433 的接触次数最多,分别有 24 种和 20 种化合物进入最终列表。通过体外 FGF23 介导的细胞外信号调节激酶 (ERK) 活性对五种化合物进行了实验评估,其中两种化合物的活性显着降低。预计这两种化合物对 α-Klotho 具有良好的结合亲和力,但与热点 Tyr433 没有大量接触。 实验发现 ZINC12409120 可以破坏 FGF23:α-Klotho 相互作用,使 FGF23 介导的 ERK 活性降低 70%,半数抑制浓度 (IC 50 ) 为 5.0 ± 0.23 μM。从计算机对接姿势开始对 ZINC12409120:α-Klotho 复合物进行的分子动力学 (MD) 模拟表明,配体同时与 KL1 结构域、KL1-KL2 连接子和 α-Klotho 的 KL2 结构域上的残基接触,从而可能会破坏 α-Klotho 的正常功能并阻碍 FGF23:α-Klotho 相互作用。 ZINC12409120 是先导化合物优化的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号