当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(I)-Catalyzed Enantioselective γ-Boryl Substitution of Trifluoromethyl- and Silyl-Substituted Alkenes
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-07-22 , DOI: 10.1002/ejoc.202200664 Natsuki Oyama 1 , Sota Akiyama 1 , Koji Kubota 1 , Tsuneo Imamoto 2 , Hajime Ito 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2022-07-22 , DOI: 10.1002/ejoc.202200664 Natsuki Oyama 1 , Sota Akiyama 1 , Koji Kubota 1 , Tsuneo Imamoto 2 , Hajime Ito 3
Affiliation
|
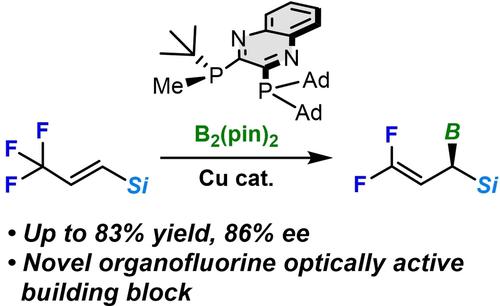
Trifluoromethyl-substituted alkenyl silanes were converted into optically active gem-difluoro-1-silyl-allylboronates by a copper(I)-catalyzed borylation utilizing optically active, C1 symmetric QuinoxP*-type bisphosphine ligands in good yield and enantioselectivity (up to 83 % yield, 86 % ee). The product of this reaction can be used as optically active organofluorine building blocks through further transformation.
中文翻译:

Cu(I)-催化三氟甲基和甲硅烷基取代烯烃的对映选择性 γ-硼烷基取代
三氟甲基取代的烯基硅烷通过铜 (I) 催化的硼化反应转化为具有光学活性的偕-二氟-1-甲硅烷基-烯丙基硼酸酯,利用光学活性的C 1对称 QuinoxP* 型双膦配体以良好的产率和对映选择性(高达 83 % 产率,86% ee)。该反应产物经进一步转化可用作光学活性有机氟结构单元。
更新日期:2022-07-22
中文翻译:

Cu(I)-催化三氟甲基和甲硅烷基取代烯烃的对映选择性 γ-硼烷基取代
三氟甲基取代的烯基硅烷通过铜 (I) 催化的硼化反应转化为具有光学活性的偕-二氟-1-甲硅烷基-烯丙基硼酸酯,利用光学活性的C 1对称 QuinoxP* 型双膦配体以良好的产率和对映选择性(高达 83 % 产率,86% ee)。该反应产物经进一步转化可用作光学活性有机氟结构单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号