当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mebendazole’s Conformational Space and Its Predicted Binding to Human Heat-Shock Protein 90
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jcim.2c00290 Walter Fiedler 1 , Fabian Freisleben 1 , Jasmin Wellbrock 1 , Karl N Kirschner 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jcim.2c00290 Walter Fiedler 1 , Fabian Freisleben 1 , Jasmin Wellbrock 1 , Karl N Kirschner 2
Affiliation
|
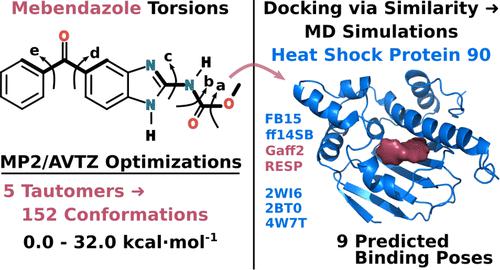
Recent experimental evidence suggests that mebendazole, a popular antiparasitic drug, binds to heat shock protein 90 (Hsp90) and inhibits acute myeloid leukemia cell growth. In this study we use quantum mechanics (QM), molecular similarity, and molecular dynamics (MD) calculations to predict possible binding poses of mebendazole to the adenosine triphosphate (ATP) binding site of Hsp90. Extensive conformational searches and minimization of the five mebendazole tautomers using the MP2/aug-cc-pVTZ theory level resulted in 152 minima. Mebendazole-Hsp90 complex models were subsequently created using the QM optimized conformations and protein coordinates obtained from experimental crystal structures that were chosen through similarity calculations. Nine different poses were identified from a total of 600 ns of explicit solvent, all-atom MD simulations using two different force fields. All simulations support the hypothesis that mebendazole is able to bind to the ATP binding site of Hsp90.
中文翻译:

甲苯咪唑的构象空间及其与人类热休克蛋白 90 的预测结合
最近的实验证据表明,流行的抗寄生虫药甲苯咪唑与热休克蛋白 90 (Hsp90) 结合并抑制急性髓细胞白血病细胞的生长。在本研究中,我们使用量子力学 (QM)、分子相似性和分子动力学 (MD) 计算来预测甲苯咪唑与 Hsp90 三磷酸腺苷 (ATP) 结合位点的可能结合位姿。使用 MP2/aug-cc-pVTZ 理论水平对五种甲苯咪唑互变异构体进行广泛的构象搜索和最小化,得到 152 个最小值。随后使用从通过相似性计算选择的实验晶体结构获得的 QM 优化构象和蛋白质坐标创建甲苯达唑-Hsp90 复合模型。从总共 600 ns 的显式溶剂中识别出九种不同的姿势,使用两个不同的力场进行全原子 MD 模拟。所有模拟都支持甲苯咪唑能够与 Hsp90 的 ATP 结合位点结合的假设。
更新日期:2022-07-22
中文翻译:

甲苯咪唑的构象空间及其与人类热休克蛋白 90 的预测结合
最近的实验证据表明,流行的抗寄生虫药甲苯咪唑与热休克蛋白 90 (Hsp90) 结合并抑制急性髓细胞白血病细胞的生长。在本研究中,我们使用量子力学 (QM)、分子相似性和分子动力学 (MD) 计算来预测甲苯咪唑与 Hsp90 三磷酸腺苷 (ATP) 结合位点的可能结合位姿。使用 MP2/aug-cc-pVTZ 理论水平对五种甲苯咪唑互变异构体进行广泛的构象搜索和最小化,得到 152 个最小值。随后使用从通过相似性计算选择的实验晶体结构获得的 QM 优化构象和蛋白质坐标创建甲苯达唑-Hsp90 复合模型。从总共 600 ns 的显式溶剂中识别出九种不同的姿势,使用两个不同的力场进行全原子 MD 模拟。所有模拟都支持甲苯咪唑能够与 Hsp90 的 ATP 结合位点结合的假设。











































 京公网安备 11010802027423号
京公网安备 11010802027423号