当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement of the Density and Viscosity of Diisopropyl Ether and 1-Alkanol Mixtures at Various Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jced.2c00263 Mohammad Almasi 1 , Zeinab Rafiee 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-07-22 , DOI: 10.1021/acs.jced.2c00263 Mohammad Almasi 1 , Zeinab Rafiee 1
Affiliation
|
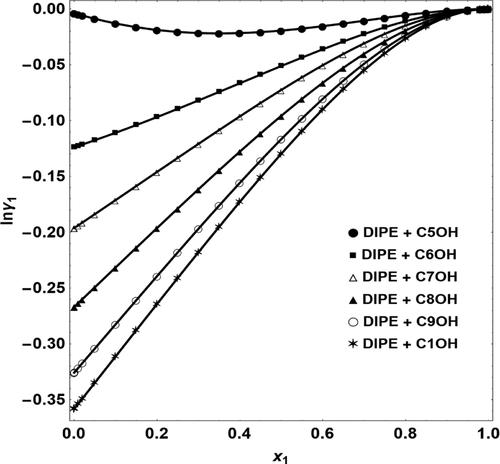
The current study was aimed to investigate intermolecular forces in six binary associating systems containing diisopropyl ether (DIPE) and normal alcohols (1-pentanol to 1-decanol) at 293.15–323.15 K. The density and viscosity of binary fluids were measured, and excess functions and partial volumes were calculated. Excess volumes and deviation in the viscosity are negative for all the above solutions. Intermolecular forces in mixtures are adjusted by molecular packing, dispersion forces, and hydrogen bonding. According to the findings, lengthening the alcohol chain increases intermolecular forces between molecules of different types. Most density and viscosity values are new and have not been reported in scientific papers yet.
中文翻译:

在不同温度下测量二异丙醚和 1-烷醇混合物的密度和粘度
目前的研究旨在研究在 293.15–323.15 K 下含有二异丙醚 (DIPE) 和正醇(1-戊醇到 1-癸醇)的六种二元缔合系统中的分子间作用力。测量二元流体的密度和粘度,以及过量计算函数和部分体积。对于上述所有溶液,过量体积和粘度偏差均为负值。混合物中的分子间作用力通过分子堆积、分散力和氢键来调节。根据研究结果,加长醇链会增加不同类型分子之间的分子间作用力。大多数密度和粘度值都是新的,尚未在科学论文中报道。
更新日期:2022-07-22
中文翻译:

在不同温度下测量二异丙醚和 1-烷醇混合物的密度和粘度
目前的研究旨在研究在 293.15–323.15 K 下含有二异丙醚 (DIPE) 和正醇(1-戊醇到 1-癸醇)的六种二元缔合系统中的分子间作用力。测量二元流体的密度和粘度,以及过量计算函数和部分体积。对于上述所有溶液,过量体积和粘度偏差均为负值。混合物中的分子间作用力通过分子堆积、分散力和氢键来调节。根据研究结果,加长醇链会增加不同类型分子之间的分子间作用力。大多数密度和粘度值都是新的,尚未在科学论文中报道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号