Minerals Engineering ( IF 4.9 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.mineng.2022.107745 M. Tang , Y. Wang , X. Niu , D. Liu
|
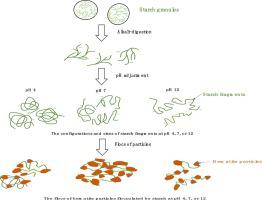
Pasting starch into sol-gels by alkali is one of the most common methods for its use as flocculants in flotation. Any variation in its gelatinization process, however, could lead to different starch solutions with certain charges, sizes, and configurations of the segments, influencing their flocculating properties onto mineral surfaces. The purpose of this study is to identify the morphological characteristics of the starch sol–gel digested with different concentrations of alkali and their flocculation mechanisms on hematite through a series of tests, such as viscosity, turbidity, adsorption, pasting titration, settling, zeta potential All results pointed out that the gel-sol transition of starch was possibly governed by alkali concentrations or starch to alkali weight ratios. As the sodium hydroxide concentration increases, an increase in viscosity but a decrease in turbidity of the starch solution was obtained, inducing an upwards trend in the settling rates of the particle flocs. A suitable sol–gel by 1.25% sodium hydroxide (a 1.6 wt ratio of starch to NaOH) at turbidity of near 2 NTU and a viscosity of 258 mL/g harvested a maximum adsorption density of 11.5 mg/g hematite at a settling rate of 7.1 × 10−5 m/s. A certain amount of acidic contents on the segment chains resulting from alkali gelatinization play an important role in determining the morphological characteristics of the starch sol–gel at different pH values affecting adsorption density on iron oxides. It is worthy to be noted that the starch remnants at very small sizes produced by high concentrations of NaOH by over 1.50% or at a starch to alkali weight ratio of less than 1.3 were hard to achieve high efficiency on flocculating the particles but occasionally dispersing them instead. It could attribute to the less possibility of “bridging interactions” between the particles and these small segments with negative charges, which may limit the size and compactness of the particle flocs, and, consequently, reduce their settling rates.
中文翻译:

淀粉溶胶-凝胶形态特征及其对细颗粒絮凝的影响
用碱将淀粉糊成溶胶凝胶是其在浮选中用作絮凝剂的最常用方法之一。然而,其糊化过程中的任何变化都可能导致不同的淀粉溶液具有某些电荷、大小和链段的构型,从而影响它们在矿物表面上的絮凝特性。本研究的目的是通过粘度、浊度、吸附、糊化滴定、沉降、zeta电位等一系列试验,确定不同浓度碱消化淀粉溶胶-凝胶的形态特征及其在赤铁矿上的絮凝机理。所有结果都指出淀粉的凝胶-溶胶转变可能受碱浓度或淀粉与碱重量比的控制。随着氢氧化钠浓度的增加,淀粉溶液的粘度增加但浊度降低,导致颗粒絮凝物的沉降速率呈上升趋势。合适的溶胶-凝胶由 1.25% 氢氧化钠(淀粉与 NaOH 的重量比为 1.6 重量比)在接近 2 NTU 的浊度和 258 mL/g 的粘度下获得的最大吸附密度为 11.5 mg/g 赤铁矿,沉降速率为7.1 × 10-5米/秒。碱糊化产生的链段上一定量的酸性含量在决定淀粉溶胶-凝胶在不同 pH 值下影响氧化铁吸附密度的形态特征中起重要作用。值得注意的是,由超过 1.50% 的高浓度 NaOH 或淀粉与碱的重量比小于 1.3 产生的极小尺寸的淀粉残留物难以实现高效的颗粒絮凝,但偶尔会分散它们反而。这可能是由于颗粒与这些带负电荷的小片段之间“桥接相互作用”的可能性较小,这可能会限制颗粒絮凝物的尺寸和致密性,从而降低它们的沉降率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号