Chemical Engineering Science ( IF 4.1 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.ces.2022.117933 Yiying Pang , Bowei Wang , Yazhuo Kang , Jiayi Li , Xiaoyi Gu , Guoqiang Kang , Xilong Yan , Yang Li , Ligong Chen
|
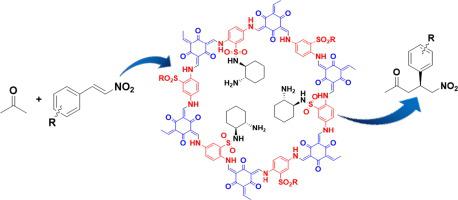
Organo-catalysts for the Michael addition reaction of nitrostyrene with acetone are essential to improve the efficiency and enantioselectivity, and their recycling also faces great challenges. Herein, a sulfonated chiral covalent organic framework (SDA-CCOF) was successfully constructed by post modification of the pre-prepared sulfonated COF with chiral 1, 2-diaminocyclohexane via N-sulfonylation. The catalytic performance was evaluated by the asymmetric Michael addition reaction of β-nitrostyrene with acetone. Under the optimized conditions, 81% conversion of β-nitrostyrene and 90% enantioselectivity were achieved. The generality of the catalyst was examined for diverse substrates with 84–96% enantioselectivity. Moreover, a possible catalytic mechanism of the Michael reaction by SDA-CCOF was speculated based on the characterization results and previous reports. This novel catalyst may provide inspirations for the development of CCOF and the loading of organo-catalysts.
中文翻译:

磺化手性共价有机骨架介导的丙酮不对称迈克尔加成β-硝基烯烃
用于硝基苯乙烯与丙酮的迈克尔加成反应的有机催化剂对于提高效率和对映选择性至关重要,它们的回收利用也面临着巨大的挑战。在此,通过N对预先制备的磺化COF与手性1, 2-二氨基环己烷进行后修饰,成功构建了磺化手性共价有机骨架(SDA - CCOF )。-磺酰化。通过β-硝基苯乙烯与丙酮的不对称迈克尔加成反应评估催化性能。在优化的条件下,β-硝基苯乙烯的转化率为 81%,对映选择性为 90%。对具有 84-96% 对映选择性的不同底物检查了催化剂的通用性。此外,基于表征结果和先前的报道,推测了SDA-CCOF的迈克尔反应的可能催化机制。这种新型催化剂可以为CCOF的发展和有机催化剂的负载提供灵感。











































 京公网安备 11010802027423号
京公网安备 11010802027423号