European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.ejmech.2022.114623 Yanghan Liu 1 , Bikash Debnath 2 , Surinder Kumar 3 , David B Lombard 3 , Nouri Neamati 2
|
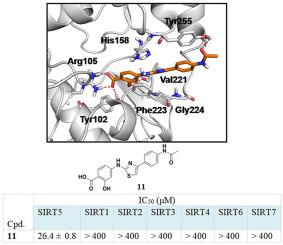
The sirtuin deacetylase SIRT5 plays important roles in regulating multiple metabolic pathways, and potentially represents an attractive target for the treatment of several human diseases, especially cancer. In this study, we report the identification of the hit compound 11 bearing a 2-hydroxybenzoic acid functional group as a novel SIRT5-selective inhibitor via our medium-throughput thermal shift screening assay. Hit 11 stabilizes SIRT5 in a dose-dependent manner and shows moderate inhibitory activity against SIRT5 and high subtype selectivity over SIRT1, 2, and 3 in a trypsin coupled enzyme-based assay. The carboxylic acid and the adjacent hydroxyl group of 11 are essential for maintaining activity. To further improve the potency of compound 11, a lead optimization was carried out, resulting in compound 43 with a 10-fold improved potency. Overall, compound 11 represents a promising new chemical scaffold for further investigation to develop SIRT5-selective inhibitors.
中文翻译:

2-羟基苯甲酸衍生物作为选择性 SIRT5 抑制剂的鉴定
sirtuin 脱乙酰酶 SIRT5 在调节多种代谢途径中起着重要作用,并可能代表治疗多种人类疾病(尤其是癌症)的有吸引力的靶标。在这项研究中,我们报告了通过我们的中等通量热转移筛选试验将带有 2-羟基苯甲酸官能团的命中化合物11鉴定为新型 SIRT5 选择性抑制剂。Hit 11以剂量依赖性方式稳定 SIRT5,并在基于胰蛋白酶偶联酶的测定中显示出对 SIRT5 的中度抑制活性和对 SIRT1、2 和 3 的高亚型选择性。11的羧酸和相邻的羟基对于维持活性至关重要。进一步提高化合物11的效力, 进行了先导优化,导致化合物43 的效力提高了 10 倍。总的来说,化合物11代表了一种有前途的新化学支架,可用于进一步研究以开发 SIRT5 选择性抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号