European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.ejmech.2022.114615 Mario Saletti 1 , Samuele Maramai 1 , Annalisa Reale 1 , Marco Paolino 1 , Simone Brogi 2 , Angela Di Capua 1 , Andrea Cappelli 1 , Gianluca Giorgi 1 , Danilo D'Avino 3 , Antonietta Rossi 3 , Carla Ghelardini 4 , Lorenzo Di Cesare Mannelli 4 , Roccaldo Sardella 5 , Andrea Carotti 5 , Gerald Woelkart 6 , Burkhard Klösch 7 , Chiara Bigogno 8 , Giulio Dondio 8 , Maurizio Anzini 1
|
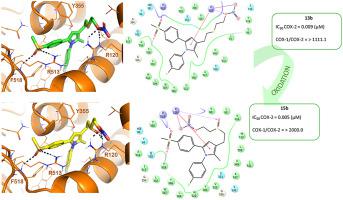
The design of compounds able to combine the selective inhibition of cyclooxygenase-2 (COX-2) with the release of nitric oxide (NO) is a promising strategy to achieve potent anti-inflammatory agents endowed with an overall safer profile and reduced toxicity upon gastrointestinal and cardiovascular systems. With the aim of generating novel and selective COX-2 inhibiting NO-donors (CINOD) and encouraged by the promising results obtained with our nitrooxy- and hydroxyethyl ethers 11 and 12 reported in previous works, we shifted our attention on the synthesis of isosteric thioanalogs nitrooxy- and hydroxy ethyl sulfides 13a-c and 14a-c, respectively, along with their oxidation products nitrooxy- and hydroxyethyl sulfoxides 15a-c and 16a-c, respectively, also referred to as thio-CINOD. Preliminary data and metabolic analysis highlighted how the isosteric substitution of the ethereal oxygen atom of 11a-c with sulfur in compounds 13a-c, independently from the presence and the number of fluorine atoms in N1-phenyl ring, leads to new selective and highly potent COX-2 inhibitors, capable to induce vasorelaxant responses in vivo. The same behavior is observed with their oxidized counterparts nitrooxyethyl sulfoxides 15a-c, in which the oxidation state of the sulfur atom and the presence of the additional oxygen atom play a substantial role in enhancing compounds activity and vasorelaxation. In addition, the screened compounds proved significantly efficacious in mouse models of inflammation and nociception at the dose of 20 mg/kg.
中文翻译:

新型镇痛/抗炎剂:1,5-二芳基吡咯硝基氧乙基硫醚和相关化合物作为环氧合酶 2 抑制剂,含有具有血管舒张特性的一氧化氮供体部分
能够将环氧合酶-2 (COX-2) 的选择性抑制与一氧化氮 (NO) 的释放相结合的化合物的设计是一种有前途的策略,可以实现具有整体安全性和降低胃肠道毒性的强效抗炎药。和心血管系统。为了产生新的和选择性的 COX-2 抑制 NO 供体 (CINOD),并受到我们先前工作中报道的硝基氧和羟乙基醚11和12所获得的有希望的结果的鼓舞,我们将注意力转移到等排硫代类似物的合成上硝基硫醚和羟乙基硫醚分别为13a-c和14a-c,以及它们的氧化产物硝基硫醚和羟乙基亚砜分别如图15a-c和16a-c所示,也称为硫代-CINOD。初步数据和代谢分析强调了化合物13a-c中11a-c的醚氧原子与硫的等排取代,与N 1 -苯环中氟原子的存在和数量无关,如何导致新的选择性和高度强效 COX-2 抑制剂,能够在体内诱导血管舒张反应。用它们的氧化对应物硝基氧乙基亚砜15a-c观察到相同的行为,其中硫原子的氧化态和额外的氧原子的存在在增强化合物活性和血管舒张方面起着重要作用。此外,筛选的化合物在 20 mg/kg 的剂量下在炎症和伤害感受的小鼠模型中证明是显着有效的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号