Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delamination by Repulsive Osmotic Swelling of Synthetic Na-Hectorite with Variable Charge in Binary Dimethyl Sulfoxide–Water Mixtures
Langmuir ( IF 3.7 ) Pub Date : 2022-07-21 , DOI: 10.1021/acs.langmuir.2c00965 Volodymyr Dudko 1 , Sabine Rosenfeldt 1 , Renée Siegel 1 , Jürgen Senker 1 , Marian Matejdes 2, 3 , Josef Breu 1
Langmuir ( IF 3.7 ) Pub Date : 2022-07-21 , DOI: 10.1021/acs.langmuir.2c00965 Volodymyr Dudko 1 , Sabine Rosenfeldt 1 , Renée Siegel 1 , Jürgen Senker 1 , Marian Matejdes 2, 3 , Josef Breu 1
Affiliation
|
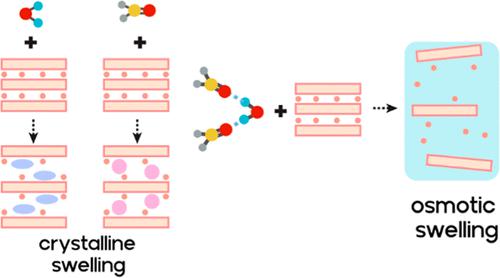
Swelling of clays is hampered by increasing layer charge. With vermiculite-type layer charge densities, crystalline swelling is limited to the two-layer hydrate, while osmotic swelling requires ion exchange with bulky and hydrophilic organic molecules or with Li+ cations to trigger repulsive osmotic swelling. Here, we report on surprising and counterintuitive osmotic swelling behavior of a vermiculite-type synthetic clay [Na0.7]inter[Mg2.3Li0.7]oct[Si4]tetO10F2 in mixtures of water and dimethyl sulfoxide (DMSO). Although swelling in pure water is restricted to crystalline swelling, with the addition of DMSO, osmotic swelling sets in at some threshold composition. Finally, when the DMSO concentration is increased further to 75 vol %, swelling is restricted again to crystalline swelling as expected. Repulsive osmotic swelling thus is observed in a narrow composition range of the binary water–DMSO mixture, where a freezing point suppression is observed. This suppression is related to DMSO and water molecules exhibiting strong interactions leading to stable molecular clusters. Based on this phenomenological observation, we hypothesize that the unexpected swelling behavior might be related to the formation of different complexes of interlayer cations being formed at different compositions. Powder X-ray diffraction and 23Na magic angle spinning-NMR evidence is presented that supports this hypothesis. We propose that the synergistic solvation of the interlayer sodium at favorable compositions exerts a steric pressure by the complexes formed in the interlayer. Concomitantly, the basal spacing is increased to a level, where entropic contributions of interlayer species lead to a spontaneous thermodynamically allowed one-dimensional dissolution of the clay stack.
中文翻译:

带可变电荷的合成钠锂蒙脱石在二元二甲亚砜-水混合物中的排斥渗透膨胀分层
粘土的膨胀受到层电荷增加的阻碍。对于蛭石型层电荷密度,结晶膨胀仅限于两层水合物,而渗透膨胀需要与体积大的亲水有机分子或 Li +阳离子进行离子交换以触发排斥性渗透膨胀。在这里,我们报告了蛭石型合成粘土 [Na 0.7 ] inter [Mg 2.3 Li 0.7 ] oct [Si 4 ] tet O 10 F 2令人惊讶和违反直觉的渗透膨胀行为在水和二甲基亚砜 (DMSO) 的混合物中。虽然纯水中的溶胀仅限于结晶溶胀,但添加 DMSO 后,渗透溶胀会在某些阈值组成处出现。最后,当 DMSO 浓度进一步增加到 75 vol% 时,溶胀再次被限制为预期的结晶溶胀。因此,在二元水-DMSO 混合物的窄组成范围内观察到排斥性渗透膨胀,其中观察到冰点抑制。这种抑制与 DMSO 和水分子表现出强烈的相互作用有关,从而导致稳定的分子簇。基于这种现象学观察,我们假设意外的溶胀行为可能与在不同组成下形成的不同层间阳离子复合物的形成有关。提出了支持这一假设的23 Na 魔角自旋核磁共振证据。我们提出,在有利的组成下,夹层钠的协同溶剂化通过在夹层中形成的配合物施加空间压力。同时,基础间距增加到一定水平,其中层间物质的熵贡献导致自发的热力学允许粘土堆的一维溶解。
更新日期:2022-07-21
中文翻译:

带可变电荷的合成钠锂蒙脱石在二元二甲亚砜-水混合物中的排斥渗透膨胀分层
粘土的膨胀受到层电荷增加的阻碍。对于蛭石型层电荷密度,结晶膨胀仅限于两层水合物,而渗透膨胀需要与体积大的亲水有机分子或 Li +阳离子进行离子交换以触发排斥性渗透膨胀。在这里,我们报告了蛭石型合成粘土 [Na 0.7 ] inter [Mg 2.3 Li 0.7 ] oct [Si 4 ] tet O 10 F 2令人惊讶和违反直觉的渗透膨胀行为在水和二甲基亚砜 (DMSO) 的混合物中。虽然纯水中的溶胀仅限于结晶溶胀,但添加 DMSO 后,渗透溶胀会在某些阈值组成处出现。最后,当 DMSO 浓度进一步增加到 75 vol% 时,溶胀再次被限制为预期的结晶溶胀。因此,在二元水-DMSO 混合物的窄组成范围内观察到排斥性渗透膨胀,其中观察到冰点抑制。这种抑制与 DMSO 和水分子表现出强烈的相互作用有关,从而导致稳定的分子簇。基于这种现象学观察,我们假设意外的溶胀行为可能与在不同组成下形成的不同层间阳离子复合物的形成有关。提出了支持这一假设的23 Na 魔角自旋核磁共振证据。我们提出,在有利的组成下,夹层钠的协同溶剂化通过在夹层中形成的配合物施加空间压力。同时,基础间距增加到一定水平,其中层间物质的熵贡献导致自发的热力学允许粘土堆的一维溶解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号