Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of pyrano[3,2-c]quinolones and furo[3,2-c]quinolones via acid-catalyzed tandem reaction of 4-hydroxy-1-methylquinolin-2(1H)-one and propargylic alcohols
RSC Advances ( IF 3.9 ) Pub Date : 2022-07-21 , DOI: 10.1039/d2ra03416f Haiting Yin 1 , Yunjun Wu 1 , Xiaoxia Gu 1 , Zhijun Feng 1 , Meifang Wang 1, 2 , Dexiang Feng 1 , Ming Wang 1 , Ziyang Cheng 1 , Shaoyin Wang 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2022-07-21 , DOI: 10.1039/d2ra03416f Haiting Yin 1 , Yunjun Wu 1 , Xiaoxia Gu 1 , Zhijun Feng 1 , Meifang Wang 1, 2 , Dexiang Feng 1 , Ming Wang 1 , Ziyang Cheng 1 , Shaoyin Wang 1, 2
Affiliation
|
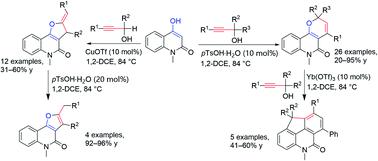
Two acid-catalyzed tandem reactions between 4-hydroxy-1-methylquinolin-2(1H)-one and propargylic alcohols are described. Depending mainly on the propargylic alcohol used, these tandem reactions proceed via either a Friedel–Crafts-type allenylation followed by 6-endo-dig cyclization sequence to form pyrano[3,2-c]quinolones or a Friedel–Crafts-type alkylation and 5-exo-dig ring closure sequence to afford furo[3,2-c]quinolones in moderate-to-high yields. The pyrano[3,2-c]quinolones products could be further transformed to tetracyclic 4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one derivatives.
中文翻译:

4-羟基-1-甲基喹啉-2(1H)-酮和炔丙醇的酸催化串联反应合成吡喃并[3,2-c]喹诺酮和呋喃[3,2-c]喹诺酮
描述了 4-hydroxy-1-methylquinolin-2(1 H )-one 和炔丙醇之间的两种酸催化串联反应。主要取决于使用的炔丙醇,这些串联反应通过Friedel-Crafts 型烯基化然后 6- endo - dig 环化序列形成吡喃并[3,2 - c ]喹诺酮类或 Friedel-Crafts 型烷基化和5- exo -dig 闭环序列以中高产率提供呋喃 [3,2- c ] 喹诺酮类药物。吡喃并[3,2 - c ]喹诺酮类产物可以进一步转化为四环4,9-二氢-5 H-环戊二烯[ lmn ]菲啶-5-酮衍生物。
更新日期:2022-07-21
中文翻译:

4-羟基-1-甲基喹啉-2(1H)-酮和炔丙醇的酸催化串联反应合成吡喃并[3,2-c]喹诺酮和呋喃[3,2-c]喹诺酮
描述了 4-hydroxy-1-methylquinolin-2(1 H )-one 和炔丙醇之间的两种酸催化串联反应。主要取决于使用的炔丙醇,这些串联反应通过Friedel-Crafts 型烯基化然后 6- endo - dig 环化序列形成吡喃并[3,2 - c ]喹诺酮类或 Friedel-Crafts 型烷基化和5- exo -dig 闭环序列以中高产率提供呋喃 [3,2- c ] 喹诺酮类药物。吡喃并[3,2 - c ]喹诺酮类产物可以进一步转化为四环4,9-二氢-5 H-环戊二烯[ lmn ]菲啶-5-酮衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号