Clinical Gastroenterology and Hepatology ( IF 11.6 ) Pub Date : 2022-07-20 , DOI: 10.1016/j.cgh.2022.07.008 John Gubatan 1 , Grant E Barber 1 , Ole Haagen Nielsen 2 , Carsten Bogh Juhl 3 , Cynthia Maxwell 4 , Michael L Eisenberg 5 , Sarah E Streett 1
|
Background & Aims
Studies evaluating reproductive outcomes among male patients with inflammatory bowel disease (IBD) are limited. We evaluated use of IBD medications and association with semen parameters, a proxy of male fertility, and adverse pregnancy outcomes (early pregnancy loss [EPL], preterm birth [PB], congenital malformations [CM]).
Methods
We searched Medline, Embase, Scopus, and Web of Science (PROSPERO CRD42020197098) from inception to April 2022 for studies reporting semen parameters and adverse pregnancy outcomes among male patients exposed to biologics, thiopurine, or methotrexate. Standardized mean difference, prevalence, and odds ratios (ORs) of outcomes were pooled and analyzed using a random effects model.
Results
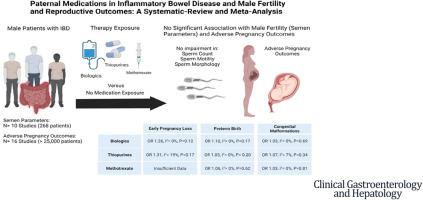
Ten studies reporting semen parameters (268 patients with IBD) and 16 studies reporting adverse pregnancy outcomes (over 25,000 patients with IBD) were included. Biologic, thiopurine, or methotrexate use were not associated with decreased sperm count, motility, or abnormal morphology compared with nonexposed patients. The prevalence of adverse pregnancy outcomes with paternal biologic (5%), thiopurine (6%), or methotrexate (6%) exposure was comparable to nonexposed patients (5%). Biologic use was not associated with risk of EPL (OR, 1.26; I2 = 0%; P = .12), PB (OR, 1.10; I2 = 0%; P = .17), or CM (OR, 1.03; I2 = 0%; P = .69). Thiopurine use was not associated with risk of EPL (OR, 1.31; I2 = 19%; P = .17), PB (OR, 1.05; I2 = 0%; P = .20), or CM (OR, 1.07; I2 = 7%; P = .34). Methotrexate use was not associated with risk of PB (OR, 1.06; I2 = 0%; P = .62) or CM (OR, 1.03; I2 = 0%; P = .81).
Conclusions
Biologic, thiopurine, or methotrexate use among male patients with IBD are not associated with impairments in fertility or with increased odds of adverse pregnancy outcomes.
中文翻译:

炎症性肠病与男性生育力和生殖结果的父亲用药:系统评价和荟萃分析
背景与目标
评估男性炎症性肠病 (IBD) 患者生殖结局的研究有限。我们评估了 IBD 药物的使用以及与精液参数、男性生育力指标和不良妊娠结局(早期流产 [EPL]、早产 [PB]、先天畸形 [CM])的关系。
方法
我们检索了 Medline、Embase、Scopus 和 Web of Science (PROSPERO CRD42020197098),从最初到 2022 年 4 月,寻找报告暴露于生物制剂、硫嘌呤或甲氨蝶呤的男性患者精液参数和不良妊娠结局的研究。使用随机效应模型汇总并分析结果的标准化均差、患病率和优势比 (OR)。
结果
其中包括 10 项报告精液参数的研究(268 名 IBD 患者)和 16 项报告不良妊娠结局的研究(超过 25,000 名 IBD 患者)。与未暴露的患者相比,生物制剂、硫嘌呤或甲氨蝶呤的使用与精子数量、活力或形态异常的减少无关。父系生物制剂 (5%)、硫嘌呤 (6%) 或甲氨蝶呤 (6%) 暴露的不良妊娠结局发生率与未暴露患者 (5%) 相当。生物制剂的使用与 EPL(OR,1.26;I 2 = 0%;P = .12)、PB(OR,1.10;I 2 = 0%;P = .17)或 CM(OR,1.03)风险无关。;I 2 = 0%;P = .69)。硫嘌呤的使用与 EPL(OR,1.31;I 2 = 19%;P = .17)、PB(OR,1.05;I 2 = 0%;P = .20)或 CM(OR,1.07)风险无关。;I 2 = 7%;P = .34)。甲氨蝶呤的使用与 PB(OR,1.06;I 2 = 0%;P = .62)或 CM(OR,1.03;I 2 = 0%;P = .81)风险无关 。
结论
男性 IBD 患者使用生物制剂、硫嘌呤或甲氨蝶呤与生育能力受损或不良妊娠结局几率增加无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号