Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2022-07-20 , DOI: 10.1016/j.aca.2022.340160 Jonas Carneiro Cruz 1 , Mariana Azevedo Rosa 2 , Lucas Morés 3 , Eduardo Carasek 3 , José Alexandre de Souza Crippa 4 , Eduardo Costa Figueiredo 2 , Maria Eugênia Costa Queiroz 1
|
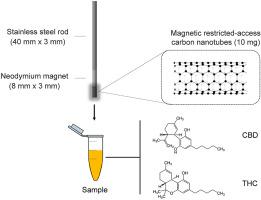
This manuscript describes the development of magnetic restricted-access carbon nanotubes (M-RACNTs) for use as SPME sorbent to determine cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) in human plasma samples by UHPLC-MS/MS. The adsorptive phase was immobilized on an SPME device by electromagnetic interactions between the M-RACNTs and a cylindrical neodymium magnet (3-mm diameter x 8-mm height) attached to a stainless-steel rod (3-mm diameter x 40-mm height). The M-RACNTs were synthesized by incorporating Fe3O4 magnetic nanoparticles (MNPs) into commercial carbon nanotubes (CNTs); then the surface of the resulting sorbent was further coated with a layer of bovine serum albumin (BSA). Characterization techniques (SEM, FTIR, and Zeta potential) confirmed the presence of both MNPs and BSA layer dispersed through the structure of the CNTs. The M-RACNTs presented adequate sorption capacity, stable physical/chemical characteristics, and appropriate magnetic properties. Protein exclusion capacity (about 98.5%) was attributed to the chemical diffusion barrier created by the BSA network at the outer surface of the sorbent. The SPME parameters (sample pH, equilibrium time, and desorption conditions) were optimized by design of experiments (fraction factorial planning). The method (validated according to the FDA guidelines) presented adequate selectivity and linearity (coefficient of determination higher than 0.99) at concentrations ranging from the lower limit of quantification (LLOQ) (10 ng mL−1) to the upper limit of quantification (ULOQ) (300 ng mL−1) for both CBD and THC. Precision and accuracy varied from 4.47 to 19.84% (LLOQ) and −6.90 to 17.78% (LLOQ), respectively. Carry-over and matrix effect were not significant. The method was successfully applied to determine plasmatic CBD levels in healthy volunteers attending a single session of oral drug administration and THC levels in frequent cannabis smokers.
中文翻译:

用于固相微萃取的磁性限制进入碳纳米管通过 UHPLC-MS/MS 测定血浆样品中的大麻素
本手稿描述了磁性限制进入碳纳米管 (M-RACNT) 的开发,用作 SPME 吸附剂,通过 UHPLC-MS/MS 测定人血浆样品中的大麻二酚 (CBD) 和 delta-9-四氢大麻酚 (THC)。通过 M-RACNT 与连接到不锈钢棒(直径 3 毫米 x 高度 40 毫米)上的圆柱形钕磁铁(直径 3 毫米 x 高度 8 毫米)之间的电磁相互作用,将吸附相固定在固相微萃取装置上)。通过掺入 Fe 3 O 4合成 M-RACNT将磁性纳米颗粒 (MNP) 制成商业碳纳米管 (CNT);然后在所得吸附剂的表面进一步涂上一层牛血清白蛋白(BSA)。表征技术(SEM、FTIR 和 Zeta 电位)证实存在分散在 CNT 结构中的 MNP 和 BSA 层。M-RACNTs表现出足够的吸附能力、稳定的物理/化学特性和适当的磁性。蛋白质排斥能力(约 98.5%)归因于 BSA 网络在吸附剂外表面产生的化学扩散屏障。SPME 参数(样品 pH 值、平衡时间和解吸条件)通过实验设计(部分因子规划)进行了优化。-1 ) 到 CBD 和 THC 的定量上限 (ULOQ) (300 ng mL -1 )。精度和准确度分别从 4.47 到 19.84% (LLOQ) 和 -6.90 到 17.78% (LLOQ) 不等。遗留效应和基质效应不显着。该方法成功地应用于确定参加单次口服药物给药的健康志愿者的血浆 CBD 水平和经常吸食大麻的人的 THC 水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号