European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-07-20 , DOI: 10.1016/j.ejmech.2022.114619 Romain Mustière 1 , Prisca Lagardère 2 , Sébastien Hutter 3 , Viviana Dell'Orco 1 , Nadia Amanzougaghene 4 , Shahin Tajeri 4 , Jean-François Franetich 4 , Sophie Corvaisier 5 , Marc Since 5 , Aurélie Malzert-Fréon 5 , Nicolas Masurier 2 , Vincent Lisowski 2 , Pierre Verhaeghe 6 , Dominique Mazier 4 , Nadine Azas 3 , Patrice Vanelle 7 , Nicolas Primas 7
|
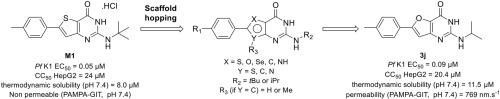
Gamhepathiopine (also known as M1), is a multi-stage acting antiplasmodial 2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one hydrochloride that was first described in 2015. The development of this compound is limited by poor microsomal stability, insufficient aqueous solubility and low intestinal permeability. In order to obtain new optimized derivatives, we conducted a scaffold hopping strategy from compound M1, resulting in the synthesis of 20 new compounds belonging to six chemical series. All the compounds were tested on the K1 multi-resistant strain of Plasmodium falciparum and the human HepG2 cell-line, to evaluate their antiplasmodial activity and their cytotoxicity. Analogues’ biological results also highlighted the mandatory presence of a heteroatom at position 5 of the thieno[3,2-d]pyrimidin-4(3H)-one moeity for the antiplasmodial activity. However, modifications at position 7 were detrimental for the antiplasmodial activity. We identified furane bioisostere 3j as a promising candidate, showing good blood stage antiplasmodial activity, better water solubility and highly improved intestinal permeability in the PAMPA assay.
中文翻译:

使用支架跳跃策略合成抗疟原虫 2-氨基噻吩并[3,2-d]嘧啶-4(3H)-one 类似物
Gamhepathiopine(也称为 M1)是一种多阶段作用的抗疟原虫 2-叔丁基氨基噻吩并[3,2 - d ]嘧啶-4(3 H )-one 盐酸盐,于 2015 年首次被描述。该化合物的开发是受到微粒体稳定性差、水溶性不足和肠道通透性低的限制。为了获得新的优化衍生物,我们对化合物 M1 进行了支架跳跃策略,从而合成了属于六个化学系列的 20 种新化合物。所有化合物均在恶性疟原虫K1 多重耐药菌株上进行了测试和人类 HepG2 细胞系,以评估它们的抗疟原虫活性和细胞毒性。类似物的生物学结果还强调了杂原子必须存在于噻吩并[3,2 - d ]嘧啶-4(3 H )-one 部分的第 5 位以实现抗疟原虫活性。然而,第 7 位的修饰对抗疟原虫活性是有害的。我们将呋喃生物等排体3j确定为有希望的候选者,在 PAMPA 测定中显示出良好的血液阶段抗疟原虫活性、更好的水溶性和高度改善的肠道通透性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号