Chem ( IF 19.1 ) Pub Date : 2022-07-20 , DOI: 10.1016/j.chempr.2022.06.014 Shuai Zhu , Jian-Hui Mao , Jun Kee Cheng , Shao-Hua Xiang , Bin Tan
|
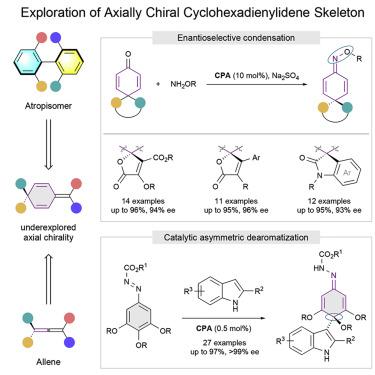
Studies of axial chirality have advanced to become an integral part of modern chemistry research, especially in asymmetric catalysis. Most investigations have revolved around catalytic enantioselective construction of the privileged axially chiral scaffolds, whereas the discovery of alternative scaffolds lags far behind. Described here is the exploitation of axially chiral molecules that are derived by substituting one C=C double bond of allenes with a cyclohexadienyl moiety. Structurally diverse analogs could be accessed in high efficiency with excellent stereocontrol through two facile and direct synthetic strategies, namely CPA-catalyzed asymmetric dearomatization strategy and enantioselective condensation strategy. Notably, the sterically bulky CPA catalyst plays a central role in the extraordinary levels of chemo-, site-, and enantioselectivities of the dearomatization strategy. These findings not only expand the current library of axially chiral compounds but also offer additional insights to explore other axially chiral scaffolds with different skeletal frameworks.
中文翻译:

轴向手性亚环己二烯骨架的发现和有机催化对映选择性构建
轴向手性研究已经发展成为现代化学研究的一个组成部分,特别是在不对称催化中。大多数研究都围绕着特殊的轴向手性支架的催化对映选择性构建展开,而替代支架的发现则远远落后。这里描述的是通过用环己二烯基部分取代丙二烯的一个 C=C 双键衍生的轴向手性分子的开发。通过两种简便直接的合成策略,即 CPA 催化的不对称脱芳构化策略和对映选择性缩合策略,可以高效地获得结构多样的类似物,并具有出色的立体控制。值得注意的是,空间庞大的 CPA 催化剂在化学、位点、和脱芳构化策略的对映选择性。这些发现不仅扩展了当前的轴向手性化合物库,而且为探索具有不同骨架的其他轴向手性支架提供了额外的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号