Cell Chemical Biology ( IF 6.6 ) Pub Date : 2022-07-18 , DOI: 10.1016/j.chembiol.2022.06.010 Ian D Ferguson 1 , Yu-Hsiu T Lin 1 , Christine Lam 1 , Hao Shao 2 , Kevin M Tharp 3 , Martina Hale 1 , Corynn Kasap 4 , Margarette C Mariano 1 , Audrey Kishishita 5 , Bonell Patiño Escobar 1 , Kamal Mandal 1 , Veronica Steri 6 , Donghui Wang 6 , Paul Phojanakong 6 , Sami T Tuomivaara 1 , Byron Hann 6 , Christoph Driessen 7 , Brian Van Ness 8 , Jason E Gestwicki 2 , Arun P Wiita 1
|
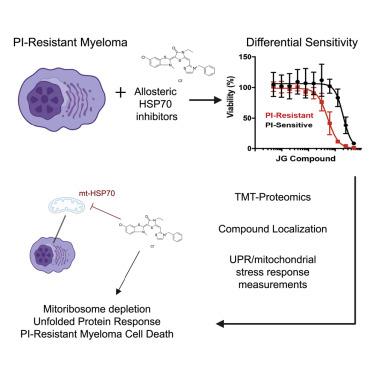
Proteasome inhibitor (PI) resistance remains a central challenge in multiple myeloma. To identify pathways mediating resistance, we first mapped proteasome-associated genetic co-dependencies. We identified heat shock protein 70 (HSP70) chaperones as potential targets, consistent with proposed mechanisms of myeloma cells overcoming PI-induced stress. We therefore explored allosteric HSP70 inhibitors (JG compounds) as myeloma therapeutics. JG compounds exhibited increased efficacy against acquired and intrinsic PI-resistant myeloma models, unlike HSP90 inhibition. Shotgun and pulsed SILAC mass spectrometry demonstrated that JGs unexpectedly impact myeloma proteostasis by destabilizing the 55S mitoribosome. Our data suggest JGs have the most pronounced anti-myeloma effect not through inhibiting cytosolic HSP70 proteins but instead through mitochondrial-localized HSP70, HSPA9/mortalin. Analysis of myeloma patient data further supports strong effects of global proteostasis capacity, and particularly HSPA9 expression, on PI response. Our results characterize myeloma proteostasis networks under therapeutic pressure while motivating further investigation of HSPA9 as a specific vulnerability in PI-resistant disease.
中文翻译:

变构 HSP70 抑制剂扰乱线粒体蛋白质稳态并克服多发性骨髓瘤中的蛋白酶体抑制剂耐药性
蛋白酶体抑制剂(PI)耐药性仍然是多发性骨髓瘤的主要挑战。为了确定介导耐药性的途径,我们首先绘制了与蛋白酶体相关的遗传相互依赖性。我们将热休克蛋白 70 (HSP70) 伴侣确定为潜在靶标,这与骨髓瘤细胞克服 PI 诱导应激的机制一致。因此,我们探索了变构 HSP70 抑制剂(JG 化合物)作为骨髓瘤治疗药物。与 HSP90 抑制不同,JG 化合物对获得性和内在 PI 耐药性骨髓瘤模型表现出增强的功效。鸟枪法和脉冲 SILAC 质谱分析表明,JG 通过破坏 55S 线粒体核糖体的稳定性,出乎意料地影响骨髓瘤蛋白质稳态。我们的数据表明,JG 具有最显着的抗骨髓瘤作用,不是通过抑制胞质 HSP70 蛋白,而是通过线粒体定位的 HSP70、HSPA9/mortalin。对骨髓瘤患者数据的分析进一步支持了整体蛋白质稳态能力,特别是HSPA9表达对 PI 反应的强烈影响。我们的结果描述了治疗压力下骨髓瘤蛋白稳态网络的特征,同时激发了对 HSPA9 作为 PI 耐药疾病的特定脆弱性的进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号