Kidney International ( IF 14.8 ) Pub Date : 2022-07-19 , DOI: 10.1016/j.kint.2022.06.022 Laura M Dember 1 , Adriana Hung 2 , Rajnish Mehrotra 3 , Jesse Y Hsu 4 , Dominic S Raj 5 , David M Charytan 6 , Finnian R Mc Causland 7 , Renu Regunathan-Shenk 5 , J Richard Landis 4 , Paul L Kimmel 8 , Alan S Kliger 9 , Jonathan Himmelfarb 10 , T Alp Ikizler 2 ,
|
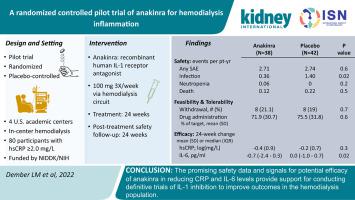
Chronic inflammation is highly prevalent among patients receiving maintenance hemodialysis and is associated with morbidity and mortality. Inhibiting inflammation with anti-cytokine therapy has been proposed but not well studied in this population. Therefore, we conducted the ACTION trial, a pilot, multicenter, randomized, placebo-controlled trial of an IL-1 receptor antagonist, anakinra, to evaluate safety, tolerability, and feasibility, and explore efficacy. Eighty hemodialysis patients with plasma concentrations of high sensitivity C-reactive protein (hsCRP) 2 mg/L and above were randomized 1:1 to placebo or anakinra 100 mg, three times per week via the hemodialysis circuit for 24 weeks, with an additional 24 weeks of post-treatment safety monitoring. Efficacy outcomes included changes in hsCRP (primary), cytokines, and patient-reported outcomes. Rates of serious adverse events and deaths were similar with anakinra and placebo (serious adverse events: 2.71 vs 2.74 events/patient-year; deaths: 0.12 vs 0.22 events/patient-year). The rate of adverse events of interest (including infections and cytopenias) was significantly lower with anakinra than placebo (0.48 vs 1.40 events/patient-year). Feasibility was demonstrated by attaining the enrollment target, a retention rate of 80%, and administration of 72% of doses. The median decrease in hsCRP from baseline to Week 24 was 41% in the anakinra group and 6% in the placebo group, a between-group difference that was not statistically significant. For IL-6, the median decreases were significant: 25% and 0% in the anakinra and placebo groups, respectively. An effect of anakinra on patient-reported outcomes was not evident. Thus, anakinra was well tolerated and did not increase infections or cytopenias. The promising safety data and potential efficacy on CRP and IL-6 provide support for conducting definitive trials of IL-1 inhibition to improve outcomes in hemodialysis patients.
中文翻译:

阿那白滞素治疗血液透析炎症的随机对照试验
慢性炎症在接受维持性血液透析的患者中非常普遍,并且与发病率和死亡率相关。已经提出用抗细胞因子疗法抑制炎症,但在该人群中尚未得到充分研究。因此,我们进行了 ACTION 试验,这是一项针对 IL-1 受体拮抗剂阿那白滞素的试点、多中心、随机、安慰剂对照试验,以评估安全性、耐受性和可行性,并探索疗效。80 名血浆超敏 C 反应蛋白 (hsCRP) 浓度为 2 mg/L 及以上的血液透析患者按 1:1 随机分配至安慰剂或阿那白滞素 100 mg,通过血液透析回路每周 3 次,持续 24 周,另外 24 周数周的治疗后安全监测。疗效结果包括 hsCRP(主要)、细胞因子和患者报告结果的变化。阿那白滞素和安慰剂的严重不良事件和死亡发生率相似(严重不良事件:2.71 比 2.74 事件/患者年;死亡:0.12 比 0.22 事件/患者年)。阿那白滞素组的不良事件发生率(包括感染和血细胞减少)显着低于安慰剂组(0.48 例事件/患者年与 1.40 例事件/患者年)。通过实现入组目标、80% 的保留率和 72% 的剂量管理证明了可行性。从基线到第 24 周,阿那白滞素组 hsCRP 的中位下降为 41%,安慰剂组为 6%,组间差异不具有统计学意义。对于 IL-6,中位下降显着:阿那白滞素组和安慰剂组分别下降 25% 和 0%。阿那白滞素对患者报告的结果的影响并不明显。因此,阿那白滞素具有良好的耐受性,不会增加感染或血细胞减少。令人鼓舞的安全性数据以及 CRP 和 IL-6 的潜在功效为开展 IL-1 抑制的明确试验提供了支持,以改善血液透析患者的预后。











































 京公网安备 11010802027423号
京公网安备 11010802027423号