Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2022-07-19 , DOI: 10.1016/j.jwpe.2022.103007 Shun Saito , Yoshihiko Matsui , Nobutaka Shirasaki , Taku Matsushita
|
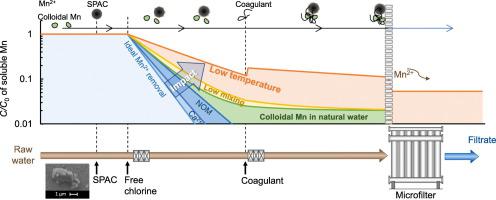
Catalytic oxidation of dissolved divalent Mn ion (Mn2+) by free chlorine and superfine powdered activated carbon (SPAC) before microfiltration has been emerged as a new technology to remove Mn from natural water for drinking water production. However, Mn was not sufficiently removed in pilot-plant experiments yet. The present study clarified underlaying removal mechanisms. In natural water, soluble Mn exists as colloidal Mn with a molecular weight ≥ 10,000 Da in the oxidized form in addition to Mn2+. Mn2+ was removed by SPAC‑chlorine, but Ca2+, Mg2+, and NOM decreased the Mn2+ removal rate as a result of competitive adsorption. The colloidal Mn was not removed by SPAC‑chlorine, but could be removed by coagulation and microfiltration or ultrafiltration alone. With the addition of coagulant (poly‑aluminum chloride), the soluble Mn concentration increased because of redissolution of precipitated-oxidized Mn due an increase in local acidity, in particular at low temperature, and coagulation reduced soluble Mn removal rate due to hindrance of mass transfer. These negative impacts may be partially attenuated by using high-intensity mixing or waiting to add the coagulant after the oxidation of Mn2+ has progressed sufficiently. Mn removal rate changed with temperature and mixing intensity in the same trend as predicted by the mass-transfer model, but it was smaller than the prediction. Together with the effects of Ca2+, Mg2+, and NOM, it appears that not only mass transfer but also the process of adsorption is a factor affecting the overall Mn removal rate.
中文翻译:

影响超细粉状活性炭和游离氯催化氧化去除天然水中可溶性锰的因素
游离氯和超细粉状活性炭(SPAC)在微滤前催化氧化溶解的二价锰离子(Mn 2+ )已成为一种从天然水中去除锰的新技术,用于生产饮用水。然而,在中试工厂实验中尚未充分去除 Mn。本研究阐明了底层去除机制。在天然水中,除Mn 2+外,可溶性Mn以氧化形式存在,分子量≥10,000 Da的胶体Mn 。SPAC-氯去除了Mn 2+ ,但 Ca 2+、Mg 2+和 NOM 降低了 Mn 2+竞争吸附导致的去除率。SPAC-氯不能去除胶体锰,但可以单独通过混凝和微滤或超滤去除。加入混凝剂(聚合氯化铝)后,可溶性锰浓度增加,这是由于局部酸度增加,沉淀氧化锰重新溶解,特别是在低温下,混凝降低了可溶性锰去除率,因为质量的阻碍转移。这些负面影响可以通过使用高强度混合或在Mn 2+氧化后等待加入混凝剂来部分减弱已经足够进步了。Mn去除率随温度和混合强度的变化趋势与传质模型预测的趋势相同,但小于预测值。连同Ca 2+、Mg 2+和NOM 的影响,似乎不仅传质而且吸附过程是影响整体Mn去除率的因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号