当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent Sites Improve Docking Performance of Protein–Protein Complexes and Protein–Protein Interface-Targeted Drugs
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.jcim.2c00264 Gonzalo F Mayol 1 , Lucas A Defelipe 1, 2 , Juan Pablo Arcon 1, 3, 4 , Adrian G Turjanski 1 , Marcelo A Marti 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.jcim.2c00264 Gonzalo F Mayol 1 , Lucas A Defelipe 1, 2 , Juan Pablo Arcon 1, 3, 4 , Adrian G Turjanski 1 , Marcelo A Marti 1
Affiliation
|
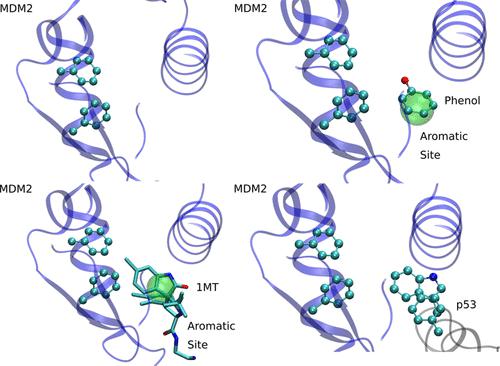
Protein–protein interactions (PPIs) are essential, and modulating their function through PPI-targeted drugs is an important research field. PPI sites are shallow protein surfaces readily accessible to the solvent, thus lacking a proper pocket to fit a drug, while their lack of endogenous ligands prevents drug design by chemical similarity. The development of PPI-blocking compounds is, therefore, a tough challenge. Mixed solvent molecular dynamics has been shown to reveal protein–ligand interaction hot spots in protein active sites by identifying solvent sites (SSs). Furthermore, our group has shown that SSs significantly improve protein–ligand docking. In the present work, we extend our analysis to PPI sites. In particular, we analyzed water, ethanol, and phenol-derived sites in terms of their capacity to predict protein–drug and protein–protein interactions. Subsequently, we show how this information can be incorporated to improve both protein–ligand and protein–protein docking. Finally, we highlight the presence of aromatic clusters as key elements of the corresponding interactions.
中文翻译:

溶剂位点提高蛋白质-蛋白质复合物和蛋白质-蛋白质界面靶向药物的对接性能
蛋白质-蛋白质相互作用 (PPI) 是必不可少的,通过 PPI 靶向药物调节其功能是一个重要的研究领域。PPI 位点是易于接近溶剂的浅蛋白质表面,因此缺乏适合药物的口袋,而它们缺乏内源性配体则阻碍了通过化学相似性进行药物设计。因此,开发 PPI 封闭化合物是一项艰巨的挑战。混合溶剂分子动力学已被证明可以通过识别溶剂位点 (SSs) 来揭示蛋白质活性位点中的蛋白质-配体相互作用热点。此外,我们小组已经表明,SSs 显着改善了蛋白质-配体对接。在目前的工作中,我们将分析扩展到 PPI 站点。特别是,我们分析了水、乙醇、和苯酚衍生位点预测蛋白质-药物和蛋白质-蛋白质相互作用的能力。随后,我们展示了如何整合这些信息以改善蛋白质-配体和蛋白质-蛋白质对接。最后,我们强调芳族簇的存在是相应相互作用的关键要素。
更新日期:2022-07-19
中文翻译:

溶剂位点提高蛋白质-蛋白质复合物和蛋白质-蛋白质界面靶向药物的对接性能
蛋白质-蛋白质相互作用 (PPI) 是必不可少的,通过 PPI 靶向药物调节其功能是一个重要的研究领域。PPI 位点是易于接近溶剂的浅蛋白质表面,因此缺乏适合药物的口袋,而它们缺乏内源性配体则阻碍了通过化学相似性进行药物设计。因此,开发 PPI 封闭化合物是一项艰巨的挑战。混合溶剂分子动力学已被证明可以通过识别溶剂位点 (SSs) 来揭示蛋白质活性位点中的蛋白质-配体相互作用热点。此外,我们小组已经表明,SSs 显着改善了蛋白质-配体对接。在目前的工作中,我们将分析扩展到 PPI 站点。特别是,我们分析了水、乙醇、和苯酚衍生位点预测蛋白质-药物和蛋白质-蛋白质相互作用的能力。随后,我们展示了如何整合这些信息以改善蛋白质-配体和蛋白质-蛋白质对接。最后,我们强调芳族簇的存在是相应相互作用的关键要素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号