当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium Solvation and Mobility in Ionic Liquid Electrolytes with Asymmetric Sulfonyl-Cyano Anion
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.jced.2c00294 Drace Penley 1 , Xiaoyu Wang 2 , Yun-Yang Lee 1 , Mounesha N. Garaga 3 , Raziyeh Ghahremani 1 , Steve Greenbaum 3 , Edward J. Maginn 2 , Burcu Gurkan 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.jced.2c00294 Drace Penley 1 , Xiaoyu Wang 2 , Yun-Yang Lee 1 , Mounesha N. Garaga 3 , Raziyeh Ghahremani 1 , Steve Greenbaum 3 , Edward J. Maginn 2 , Burcu Gurkan 1
Affiliation
|
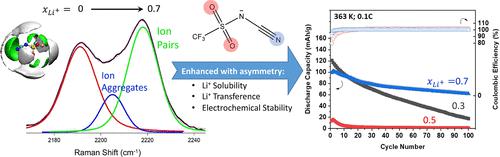
The solvation structure and transport properties of Li+ in ionic liquid (IL) electrolytes based on n-methyl-n-butylpyrrolidinium cyano(trifluoromethanesulfonyl)imide [PYR14][CTFSI] and [Li][CTFSI] (0 ≤ xLi ≤ 0.7) were studied by Raman and Nuclear Magnetic Resonance (NMR) diffusometry, and molecular dynamics (MD) simulations. At xLi < 0.3, Li+ coordination is dominated by the cyano group. As xLi is increased, free cyano-sites become limited, resulting in increased coordination via the sulfonyl group. The 1:1 mixture of the symmetric anions bis(trifluoromethanesulfonyl)imide ([TFSI]) and dicyanamide ([DCA]) results in similar physical properties as the IL with [CTFSI]. However, anion asymmetry is shown to increase Li-salt solubility and promote Li+ transference. The lifetimes of Li+-cyano coordination for [CTFSI] are calculated to be shorter than those for [DCA], indicating that the competition from the sulfonyl group weakens its solvation with Li+. This resulted in higher Li+ transference for the electrolyte with [CTFSI]. In relation to the utility of these electrolytes in energy storage, the Li–LiFePO4 half cells assembled with IL electrolyte (xLi = 0.3, 0.5, and 0.7) demonstrated a nominal capacity of 140 mAh/g at 0.1C rate and 90 °C where the cell with xLi = 0.7 IL electrolyte demonstrated 61% capacity retention after 100 cycles and superior rate capability owing to increased electrochemical stability.
中文翻译:

不对称磺酰基-氰基阴离子在离子液体电解质中的锂溶剂化和迁移率
基于正甲基正丁基吡咯烷氰基(三氟甲磺酰基)亚胺[PYR14][CTFSI]和[Li][CTFSI]的离子液体(IL)电解质中Li +的溶剂化结构和输运性质(0 ≤ x Li ≤ 0.7 ) 通过拉曼和核磁共振 (NMR) 扩散测量以及分子动力学 (MD) 模拟进行了研究。在x Li < 0.3 时,Li +配位由氰基支配。作为x李增加,游离氰基位点变得有限,导致通过磺酰基增加配位。对称阴离子双(三氟甲磺酰基)亚胺 ([TFSI]) 和双氰胺 ([DCA]) 的 1:1 混合物产生与具有 [CTFSI] 的 IL 相似的物理性质。然而,阴离子不对称性被证明会增加锂盐的溶解度并促进锂离子的迁移。[CTFSI]的 Li + -氰基配位寿命计算得比 [DCA] 的短,表明来自磺酰基的竞争削弱了其与 Li +的溶剂化。这导致具有[CTFSI]的电解质的Li +迁移率更高。关于这些电解质在能量存储中的应用,Li-LiFePO 4用 IL 电解质( x Li = 0.3、0.5 和 0.7)组装的半电池在 0.1C 倍率和 90 °C 下表现出 140 mAh/g 的标称容量,其中x Li = 0.7 IL 电解质的电池在之后表现出 61% 的容量保持率由于提高了电化学稳定性,100 次循环和优异的倍率性能。
更新日期:2022-07-19
中文翻译:

不对称磺酰基-氰基阴离子在离子液体电解质中的锂溶剂化和迁移率
基于正甲基正丁基吡咯烷氰基(三氟甲磺酰基)亚胺[PYR14][CTFSI]和[Li][CTFSI]的离子液体(IL)电解质中Li +的溶剂化结构和输运性质(0 ≤ x Li ≤ 0.7 ) 通过拉曼和核磁共振 (NMR) 扩散测量以及分子动力学 (MD) 模拟进行了研究。在x Li < 0.3 时,Li +配位由氰基支配。作为x李增加,游离氰基位点变得有限,导致通过磺酰基增加配位。对称阴离子双(三氟甲磺酰基)亚胺 ([TFSI]) 和双氰胺 ([DCA]) 的 1:1 混合物产生与具有 [CTFSI] 的 IL 相似的物理性质。然而,阴离子不对称性被证明会增加锂盐的溶解度并促进锂离子的迁移。[CTFSI]的 Li + -氰基配位寿命计算得比 [DCA] 的短,表明来自磺酰基的竞争削弱了其与 Li +的溶剂化。这导致具有[CTFSI]的电解质的Li +迁移率更高。关于这些电解质在能量存储中的应用,Li-LiFePO 4用 IL 电解质( x Li = 0.3、0.5 和 0.7)组装的半电池在 0.1C 倍率和 90 °C 下表现出 140 mAh/g 的标称容量,其中x Li = 0.7 IL 电解质的电池在之后表现出 61% 的容量保持率由于提高了电化学稳定性,100 次循环和优异的倍率性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号