当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Conformational Transition Pathways and Hidden Intermediates in DFG-Flip Process of c-Met Kinase Revealed by Metadynamics Simulations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-18 , DOI: 10.1021/acs.jcim.2c00770 Tao Jiang 1 , Zhenhao Liu 1 , Wenlang Liu 1 , Jiawen Chen 2 , Zheng Zheng 1 , Mojie Duan 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-18 , DOI: 10.1021/acs.jcim.2c00770 Tao Jiang 1 , Zhenhao Liu 1 , Wenlang Liu 1 , Jiawen Chen 2 , Zheng Zheng 1 , Mojie Duan 2
Affiliation
|
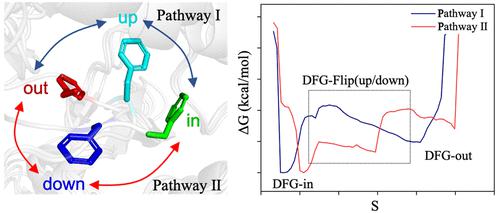
Protein kinases intrinsically translate their conformations between active and inactive states, which is key to their enzymatic activities. The conformational flipping of the three-residue conservative motif, Asp-Phe-Gly (DFG), is crucial for many kinases’ biological functions. Obtaining a detailed demonstration of the DFG flipping process and its corresponding dynamical and thermodynamical features could broaden our understanding of kinases’ conformation-activity relationship. In this study, we employed metadynamics simulation, a widely used enhanced sampling technique, to analyze the conformational transition pathways of the DFG flipping for the c-Met kinase. The corresponding free energy landscape suggested two distinct transition pathways between the “DFG-in” and “DFG-out” states of the DFG-flip from c-Met. On the basis of the orientation direction of the F1223 residue, we correspondingly named the two pathways the “DFG-up” path, featuring forming a commonly discovered “DFG-up” transition state, and the “DFG-down” path, a unique transition pathway with F1223 rotating along the opposite direction away from the hydrophobic cavity. The free energies along the two pathways were then calculated using the Path Collective Variable (PCV) metadynamics simulation. The simulation results showed that, though having similar free energy barriers, the free energy cuve for the DFG-down path suggested a two-step conformational transition mechanism, while that for the DFG-up path showed the one-step transition feature. The c-Met DFG flipping mechanism and the new intermediate state discovered in this work could provide a deeper understanding of the conformation-activity relationship for c-Met and, possibly, reveal a new conformational state as the drug target for c-Met and other similar kinases.
中文翻译:

元动力学模拟揭示c-Met激酶DFG-Flip过程中的构象转变途径和隐藏中间体
蛋白激酶本质上在活性和非活性状态之间转换其构象,这是其酶活性的关键。三残基保守基序 Asp-Phe-Gly (DFG) 的构象翻转对许多激酶的生物学功能至关重要。获得 DFG 翻转过程及其相应的动力学和热力学特征的详细演示可以拓宽我们对激酶构象-活性关系的理解。在这项研究中,我们采用元动力学模拟(一种广泛使用的增强采样技术)来分析 DFG 翻转 c-Met 激酶的构象转变途径。相应的自由能景观表明来自 c-Met 的 DFG-flip 的“DFG-in”和“DFG-out”状态之间存在两条不同的过渡路径。根据 F1223 残基的取向方向,我们将这两条路径分别命名为“DFG-up”路径,形成常见的“DFG-up”过渡态,以及“DFG-down”路径,形成独特的F1223 沿相反方向旋转远离疏水腔的过渡途径。然后使用路径集体变量 (PCV) 元动力学模拟计算沿两条路径的自由能。模拟结果表明,尽管自由能势垒相似,但DFG-down路径的自由能曲线表明了两步构象转变机制,而DFG-up路径的自由能曲线显示了一步转变特征。
更新日期:2022-07-18
中文翻译:

元动力学模拟揭示c-Met激酶DFG-Flip过程中的构象转变途径和隐藏中间体
蛋白激酶本质上在活性和非活性状态之间转换其构象,这是其酶活性的关键。三残基保守基序 Asp-Phe-Gly (DFG) 的构象翻转对许多激酶的生物学功能至关重要。获得 DFG 翻转过程及其相应的动力学和热力学特征的详细演示可以拓宽我们对激酶构象-活性关系的理解。在这项研究中,我们采用元动力学模拟(一种广泛使用的增强采样技术)来分析 DFG 翻转 c-Met 激酶的构象转变途径。相应的自由能景观表明来自 c-Met 的 DFG-flip 的“DFG-in”和“DFG-out”状态之间存在两条不同的过渡路径。根据 F1223 残基的取向方向,我们将这两条路径分别命名为“DFG-up”路径,形成常见的“DFG-up”过渡态,以及“DFG-down”路径,形成独特的F1223 沿相反方向旋转远离疏水腔的过渡途径。然后使用路径集体变量 (PCV) 元动力学模拟计算沿两条路径的自由能。模拟结果表明,尽管自由能势垒相似,但DFG-down路径的自由能曲线表明了两步构象转变机制,而DFG-up路径的自由能曲线显示了一步转变特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号