当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Second and Outer Coordination Sphere Effects in Nitrogenase, Hydrogenase, Formate Dehydrogenase, and CO Dehydrogenase
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-07-18 , DOI: 10.1021/acs.chemrev.1c00914 Sven T Stripp 1 , Benjamin R Duffus 2 , Vincent Fourmond 3 , Christophe Léger 3 , Silke Leimkühler 2 , Shun Hirota 4 , Yilin Hu 5 , Andrew Jasniewski 5 , Hideaki Ogata 4, 6, 7 , Markus W Ribbe 5, 8
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-07-18 , DOI: 10.1021/acs.chemrev.1c00914 Sven T Stripp 1 , Benjamin R Duffus 2 , Vincent Fourmond 3 , Christophe Léger 3 , Silke Leimkühler 2 , Shun Hirota 4 , Yilin Hu 5 , Andrew Jasniewski 5 , Hideaki Ogata 4, 6, 7 , Markus W Ribbe 5, 8
Affiliation
|
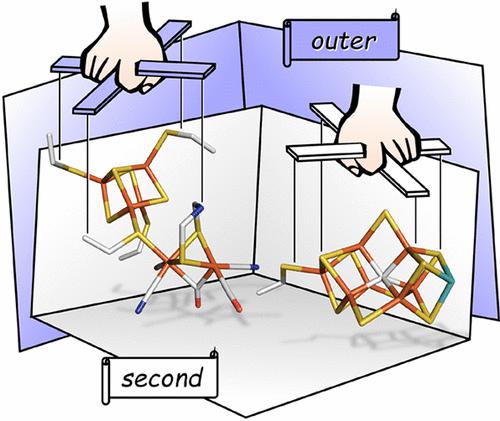
Gases like H2, N2, CO2, and CO are increasingly recognized as critical feedstock in “green” energy conversion and as sources of nitrogen and carbon for the agricultural and chemical sectors. However, the industrial transformation of N2, CO2, and CO and the production of H2 require significant energy input, which renders processes like steam reforming and the Haber-Bosch reaction economically and environmentally unviable. Nature, on the other hand, performs similar tasks efficiently at ambient temperature and pressure, exploiting gas-processing metalloenzymes (GPMs) that bind low-valent metal cofactors based on iron, nickel, molybdenum, tungsten, and sulfur. Such systems are studied to understand the biocatalytic principles of gas conversion including N2 fixation by nitrogenase and H2 production by hydrogenase as well as CO2 and CO conversion by formate dehydrogenase, carbon monoxide dehydrogenase, and nitrogenase. In this review, we emphasize the importance of the cofactor/protein interface, discussing how second and outer coordination sphere effects determine, modulate, and optimize the catalytic activity of GPMs. These may comprise ionic interactions in the second coordination sphere that shape the electron density distribution across the cofactor, hydrogen bonding changes, and allosteric effects. In the outer coordination sphere, proton transfer and electron transfer are discussed, alongside the role of hydrophobic substrate channels and protein structural changes. Combining the information gained from structural biology, enzyme kinetics, and various spectroscopic techniques, we aim toward a comprehensive understanding of catalysis beyond the first coordination sphere.
中文翻译:

固氮酶、氢化酶、甲酸脱氢酶和 CO 脱氢酶中的第二配位层和外配位层效应
H 2 、N 2 、CO 2和CO 等气体越来越被认为是“绿色”能源转换的关键原料以及农业和化学领域的氮和碳来源。然而,N 2 、CO 2和CO的工业转化以及H 2的生产需要大量的能量输入,这使得蒸汽重整和哈伯-博世反应等过程在经济和环境上都不可行。另一方面,大自然在环境温度和压力下有效地执行类似的任务,利用气体处理金属酶 (GPM) 结合基于铁、镍、钼、钨和硫的低价金属辅助因子。研究此类系统是为了了解气体转化的生物催化原理,包括固氮酶的N 2固定和氢化酶的H 2生产以及甲酸脱氢酶、一氧化碳脱氢酶和固氮酶的CO 2和CO转化。在这篇综述中,我们强调了辅因子/蛋白质界面的重要性,讨论了第二和外配位层效应如何决定、调节和优化 GPM 的催化活性。这些可能包括第二配位球中的离子相互作用,其形成辅因子上的电子密度分布、氢键变化和变构效应。在外部配位层中,讨论了质子转移和电子转移,以及疏水底物通道和蛋白质结构变化的作用。 结合从结构生物学、酶动力学和各种光谱技术中获得的信息,我们的目标是全面了解第一配位范围之外的催化作用。
更新日期:2022-07-18
中文翻译:

固氮酶、氢化酶、甲酸脱氢酶和 CO 脱氢酶中的第二配位层和外配位层效应
H 2 、N 2 、CO 2和CO 等气体越来越被认为是“绿色”能源转换的关键原料以及农业和化学领域的氮和碳来源。然而,N 2 、CO 2和CO的工业转化以及H 2的生产需要大量的能量输入,这使得蒸汽重整和哈伯-博世反应等过程在经济和环境上都不可行。另一方面,大自然在环境温度和压力下有效地执行类似的任务,利用气体处理金属酶 (GPM) 结合基于铁、镍、钼、钨和硫的低价金属辅助因子。研究此类系统是为了了解气体转化的生物催化原理,包括固氮酶的N 2固定和氢化酶的H 2生产以及甲酸脱氢酶、一氧化碳脱氢酶和固氮酶的CO 2和CO转化。在这篇综述中,我们强调了辅因子/蛋白质界面的重要性,讨论了第二和外配位层效应如何决定、调节和优化 GPM 的催化活性。这些可能包括第二配位球中的离子相互作用,其形成辅因子上的电子密度分布、氢键变化和变构效应。在外部配位层中,讨论了质子转移和电子转移,以及疏水底物通道和蛋白质结构变化的作用。 结合从结构生物学、酶动力学和各种光谱技术中获得的信息,我们的目标是全面了解第一配位范围之外的催化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号