Journal of Ecology ( IF 5.3 ) Pub Date : 2022-07-16 , DOI: 10.1111/1365-2745.13966 Emily B Bruns 1 , Michael E Hood 2 , Janis Antonovics 3 , Indigo H Ballister 3 , Sarah E Troy 4 , Jae-Hoon Cho 5
|
1 INTRODUCTION
Population age structure plays a critical role in pathogen dynamics (Anderson & May, 1985; Katzmann & Dietz, 1984), with juveniles often playing an outsized role in pathogen transmission (Altizer et al., 2004; Goldstein et al., 2018; Grenfell & Anderson, 1985; Härkönen et al., 2007; Manlove et al., 2016). For example, in humans prior to vaccination, the spread of measles and chicken pox were largely driven by school-aged children (Anderson & May, 1985; Collins, 1929; Grenfell & Anderson, 1985). Similar juvenile-biased transmission dynamics have also been reported for diseases in livestock (Brooks-Pollock et al., 2013; Klinge et al., 2009) and wildlife (Altizer et al., 2004; Härkönen et al., 2007; Manlove et al., 2016). Indeed, there is mounting evidence that the transmission of several emerging zoonotic infections of humans is driven by birth pulses of juveniles of the animal reservoir species (Amman et al., 2012; Hayman, 2015). In plants, diseases that specialize on seedling stages can influence competitive outcomes and are a critical driver of plant community diversity (Augspurger, 1984; Bever et al., 2015; Hendrix & Campbell, 1973; Mordecai, 2011; Packer & Clay, 2000). Understanding the ecological and evolutionary processes that give rise to these juvenile-biased transmission patterns is therefore critically important.
One likely reason why juveniles may be so important to pathogen transmission is that they are often less resistant (more susceptible) to infection than adults. Reviews of age-dependent resistance in plants (Develey-Rivière & Galiana, 2007), invertebrates (Ben-Ami, 2019) and our own reviews of the vertebrate literature (Table 1) show a strong pattern of increasing resistance with age. While this pattern of age-dependent resistance has long been appreciated by animal epidemiologists and plant pathologists alike, the question of why these patterns have evolved has largely been overlooked. Certainly for organisms with adaptive immune systems, the pattern of increasing resistance with age is expected, as individuals build up their antibody-mediated immune arsenal through prior exposures; however, even among vertebrates, controlled inoculation studies (where prior exposure can be excluded) tend to show that infection rates and disease symptoms decline with age (Table 1). Moreover, the same pattern of increasing resistance with age has been widely observed in inoculation studies of plants and invertebrates that rely strongly on innate immunity (Table 1, and also Reviewed in Ben-Ami, 2019; Develey-Rivière & Galiana, 2007). So why do so many organisms invest more in disease resistance at the adult stage?
| Host | Pathogen(s) | Decrease in infection or severity with age? | Result | Citation |
|---|---|---|---|---|
| Vertebrates | ||||
| Guinea pigs | Bovine Tuberculosis | Yes | Mortality of newborn guinea pigs is significantly higher than 15-day old pigs and adult pigs | (Duca, 1948) |
| Guinea pigs | Human and Bovine Tuberculosis | Yes | Highest mortality in newborn guinea pigs | (Francis, 1961) |
| Rats | Streptococcus (group B, type II) | Yes | Death rate of 1-day-old rats 85% higher than 7-day-old rats | (Zeligs et al., 1982) |
| Pigs | Porcine reproductive and respiratory syndrome virus (PRRSV) | Yes | Higher viremia and disease severity in piglets than mature pigs | (Klinge et al., 2009) |
| Mice | Mouse Mumps virus | Yes | High disease severity and mortality in 1- and 3-day-old mice than in than 7-day-old mice | (Overman, 1954) |
| Mice | Murine papovavirus (K) | Yes | High mortality in animals infected before 8 days of age, and no mortality following | (Greenlee, 1981) |
| Rats | Plasmodium berghei | Yes | Mortality was highest in youngest age group (14–17 days) and declined with age | (Zuckerman & Yoeli, 1954) |
| Invertebrates | ||||
| Daphnia magna (Water flea) | Pasteuria ramosa | Yes | Infection rate highest in 0–1-day-old Daphnia compared with older individuals | (Garbutt et al., 2014)* |
| Penaeus vannamei (white shrimp) | Baculovirus penaei | Yes | Decrease in mortality and infection with increasing age | (Leblanc & Overstreet, 1990) |
| Zootermopsis angusticollis (Termites) | Metarhizium anisopliae | Yes | Younger instars had higher mortality than older instars and nymphs | (Rosengaus & Traniello, 2001) |
| Anticarsia gemmataliss (Velvetbean caterpillar) | Nucleopolyhedro virus | Yes | LD 50 increased with age. Second instars 40% more susceptible than all other ages | (Boucias et al., 1980) |
| Glossina morsitans morsitans (Tsetse fly) | Trypanosoma congolense and T. brucei brucei | Yes | Infection highest in adult flies 0–1 days post-emergence and declined with age | (Kubi et al., 2006)* |
| Ploida interpunctella (Indian meal mouth caterpillar) | Granulosis virus | Yes | Mortality highest in youngest instar and decreased with age, even when weight was accounted for | (Sait et al., 1994)* |
| Apis mellifera (Honeybee) | Foulbrood (Bacillus larvae) | Yes | Larval mortality declined with inoculation age in both susceptible and resistant lines of honeybee larvae | (Bambrick & Rothenbuhler, 1961) |
| Biomphalaria glabrata (snail) | Schistosoma mansoni | No | Infection rate decreases with host size but is not affected by host age when size is accounted for | (Anderson et al., 1982)* |
| Danaus plexippus (monarch butterfly) | Ophryocystis elektroscirrha | No | first instar larvae had a lower infection rate than second instar larvae. Younger instars that were infected produced fewer spores |
(de Roode et al., 2006) |
| Plants | ||||
| Broccoli | Downy mildew (Hyaloperonospora parasitica) | Yes | Variable resistance to infection at the cotyledon stage among cultivars but increasing resistance in all tested cultivars at adult stage | (Coelho et al., 2009) |
| Cucumber | Pythium | Yes | Cucumber seedings inoculated at older ages were more resistant to pythium infection | (McClure & Robbins, 1942) |
| Oats | Blumeria gramminis (powdery mildew) | Yes | Adult plant resistance is well-established and takes the form of Papillae formation. Stronger in older leaves and older plants | (Sánchez-Martín et al., 2011) |
| Peanuts | Puccinia arachidis | Yes | Infection efficiency decreases and latent period increases with leaf age | (Savary, 1987) |
| Potato | Potato virus PVY | Yes | Higher infection rate in young plants, declines to zero with older plants | (Gibson, 1991) |
| Potato | Phytopthora infestans (late blight) | Yes | Susceptibility declined significantly from 3 to 6 weeks of age | (Stewart et al., 1983) |
| Snap dragon | Pythium ultimum | Yes | Strong mortality at seedling stage, reduced if inoculated 20 days after planting | (Mellano et al., 1970) |
| Wheat | Wheat dwarf virus | Yes | Strong decline in susceptibility with age | (Lindblad & Sigvald, 2004) |
| Winter wheat | Coprinus psychromorbidus (Cottony snow mould) | Yes | Older plants that had a longer period of hardening off were more resistant to infection and mortality | (Gaudet & Chen, 1987) |
Variation in disease exposure risk could be an important driver of resistance evolution at different ages. Given that resistance is often costly (Biere & Antonovics, 1996; Cotter et al., 2004; Eraud et al., 2009; Freitak et al., 2003; Tian et al., 2003), selection should favour the expression of resistance mechanisms that correspond with exposure risk at a given age, modulated by the cost (Ashby & Bruns, 2018; Bruns, 2019). For example, sexually immature juveniles are unlikely to encounter sexually transmitted diseases, and we would therefore expect selection to favour a later developmental onset of resistance to these types of diseases. In many organisms, older individuals cover more territory and consume more food, increasing their exposure to ingesting orally transmitted pathogens (Elliot et al., 2002; Garbutt et al., 2014). In social animals, adults often have higher contact rates, and higher network connectivity than juveniles (Carter et al., 2013; Rimbach et al., 2015), potentially increasing the risk of directly transmitted diseases. In contrast, adults are obviously at lower risk of vertically transmitted diseases, such as Wolbachia, although inoculation studies have shown that adults can be infected (Werren et al., 2008).
A key question is whether, and to what extent, hosts have the capacity to respond to these age-specific differences in selection. Can resistance evolve independently at adult and juvenile stages? To date, although the phenomenon of age dependence of resistance is well documented, we have little information on its underlying genetics. It has been hypothesized that developmental resource constraints at the juvenile stage could strongly limit the capacity of hosts to evolve resistance at that stage (Boege & Marquis, 2005; McDade, 2003). However, standing genetic variation for disease at the juvenile stage has been well documented in humans (Hill et al., 1991; Mockenhaupt et al., 2006), other animals (Cotter et al., 2004; Gauly et al., 2002) and plants (Chung et al., 2012; Jarosz & Burdon, 1990), indicating that host populations have the capacity to evolve resistance at young ages. Yet, we have far less information on the degree to which juvenile and adult resistances are correlated. In Drosophila melanogaster, Lesser et al. (2006) found that there was no correlation in the abilities of individual inbred lines to clear E.coli infections at 1 and 4 weeks of age post-eclosion, suggesting different loci underlie disease resistance at these two ages. In wheat and other grain crops, genome wide association studies have shown varying degrees of overlap in loci that underlie resistance to rust fungi at the seedling and adult stages (Gao et al., 2016; Liu et al., 2017; Panter & Jones, 2002; Zegeye et al., 2014). However, resistance genetics of crop plants have been strongly shaped by selective breeding and may not be an accurate reflection of evolutionary processes in nature.
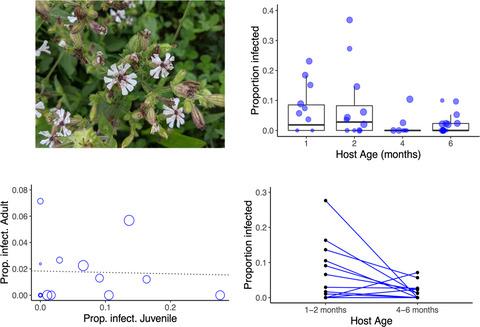
Here, we investigated the genetic correlation between seedling and adult resistance to a naturally occurring fungal disease (anther smut caused by species of Microbotryum fungi) in three wild plant species, Silene latifolia, Silene vulgaris and Dianthus pavonius. In these hosts, infection with Microbotryum causes the flowers to produce spores from the anthers, in place of pollen, and the ovaries to abort, resulting in the complete sterility of the plant (Alexander & Maltby, 1990). We showed in previous studies with D. pavonius that adult plants were much more resistant to infection by inoculation than seedlings (Bruns et al., 2017). Moreover, transmission to seedlings is a major driver of disease dynamics, and our epidemiological models of a highly diseased population indicate that 80% of transmission events occurred via seedling infection (Bruns et al., 2017). These results suggest a strong ecological importance of age-dependent resistance variation in this system. One potential reason is that selection for resistance is stronger at the adult stage due to the increased exposure risk posed by flowering (and visitation by spore-bearing pollinators). Juveniles can still encounter the pathogen through passive, aerial deposition of spores from nearby diseased plants, but this transmission mode has a much steeper dispersal gradient (Bruns et al., 2017).
In this study, we used inoculation experiments to investigate the correlations between family-level resistance at juvenile and adult stages within not only D. pavonius, but also two other host species commonly affected by anther smut, with the goal of assessing the generality of age-specific genetic differences in resistance. Strong genetic correlations would indicate that the same, or linked loci likely underlie resistance at both ages, constraining an independent response to selection for disease resistance at juvenile and adult stages. In contrast, weak correlations and strong age × family interactions would indicate that different loci underlie resistance at juvenile and adult stages, with potential for independent evolution of age-specific defence. In one species, S. latifolia, we also carried out follow-up experiments to distinguish the effects of age per se versus developmental stage (vegetative vs reproductive) on resistance.
中文翻译:

不同年龄段的抗病能力能否独立进化?三种野生植物年龄依赖性抗病性的遗传变异
1 简介
人口年龄结构在病原体动态中起着至关重要的作用(Anderson & May, 1985 ;Katzmann & Dietz, 1984 ),其中青少年往往在病原体传播中发挥着巨大作用(Altizer et al., 2004 ;Goldstein et al., 2018 ;Grenfell) & 安德森, 1985 ;Härkönen 等, 2007 ;Manlove 等, 2016 )。例如,在接种疫苗之前的人类中,麻疹和水痘的传播主要是由学龄儿童推动的(Anderson & May, 1985 ;Collins, 1929 ;Grenfell & Anderson, 1985 )。家畜疾病(Brooks-Pollock et al., 2013 ;Klinge et al., 2009 )和野生动物(Altizer et al., 2004 ;Härkönen et al., 2007 ;Manlove et al., 2007)也报告了类似的青少年偏见传播动态。等, 2016 )。事实上,越来越多的证据表明,人类几种新出现的人畜共患感染的传播是由动物宿主物种幼体的出生脉冲驱动的(Amman 等人, 2012 年;Hayman, 2015 年)。在植物中,专门针对幼苗阶段的疾病可以影响竞争结果,并且是植物群落多样性的关键驱动因素(Augspurger, 1984 ;Bever 等, 2015 ;Hendrix & Campbell, 1973 ;Mordecai, 2011 ;Packer & Clay, 2000 ) 。因此,了解产生这些青少年偏见传播模式的生态和进化过程至关重要。
青少年对病原体传播如此重要的一个可能原因是,他们通常比成年人对感染的抵抗力较低(更容易受到感染)。对植物(Develey-Rivière & Galiana, 2007 )、无脊椎动物(Ben-Ami, 2019 )的年龄依赖性抗性的回顾以及我们自己对脊椎动物文献的评论(表1)显示了随着年龄的增长抗性增加的强烈模式。虽然动物流行病学家和植物病理学家长期以来都认识到这种年龄依赖性耐药性模式,但这些模式为何进化的问题在很大程度上被忽视了。当然,对于具有适应性免疫系统的生物体来说,抵抗力随着年龄的增长而增加的模式是预期的,因为个体通过先前的暴露建立了抗体介导的免疫库。然而,即使在脊椎动物中,对照接种研究(可以排除先前的暴露)往往表明感染率和疾病症状随着年龄的增长而下降(表1)。此外,在强烈依赖先天免疫的植物和无脊椎动物的接种研究中,也广泛观察到随着年龄的增长抵抗力增加的相同模式(表 1,Ben-Ami, 2019 年也进行了综述;Develey-Rivière 和 Galiana, 2007 年)。那么,为什么这么多生物体在成年阶段会在抗病能力上投入更多呢?
表 1.测试宿主抵抗力与年龄的函数关系的研究示例。仅包括在多个宿主年龄下测量感染率或疾病严重程度的直接接种研究。有关所用搜索词的更多详细信息,请参阅补充材料。 Ben-Ami ( 2019 ) 的评论中也引用了带有星号的研究。
| 主持人 | 病原体 | 随着年龄的增长,感染程度或严重程度会降低吗? |
结果 | 引文 |
|---|---|---|---|---|
| 脊椎动物 | ||||
| 豚鼠 | 牛结核病 | 是的 | 新生豚鼠死亡率明显高于15日龄猪和成年猪 |
(杜卡, 1948 ) |
| 豚鼠 | 人类和牛结核病 |
是的 | 新生豚鼠死亡率最高 |
(弗朗西斯, 1961 ) |
| 老鼠 | 链球菌( B 组,II 型) |
是的 | 1日龄大鼠死亡率比7日龄大鼠高85% |
(泽利格斯等人, 1982 ) |
| 猪 | 猪繁殖与呼吸综合征病毒(PRRSV) |
是的 | 仔猪的病毒血症和疾病严重程度高于成年猪 |
(克林格等人, 2009 ) |
| 小鼠 | 鼠腮腺炎病毒 | 是的 | 1 日龄和 3 日龄小鼠的疾病严重程度和死亡率高于 7 日龄小鼠 |
(奥弗曼, 1954 ) |
| 小鼠 | 鼠乳多空病毒 (K) | 是的 | 8 日龄前感染的动物死亡率较高,但随后无死亡 |
(格林利, 1981 ) |
| 老鼠 | 伯氏疟原虫 | 是的 | 最年轻年龄组(14-17 天)的死亡率最高,并随着年龄的增长而下降 |
(祖克曼和约利, 1954 年) |
| 无脊椎动物 | ||||
Daphnia magna (水蚤) |
巴氏杆菌 | 是的 | 与年长个体相比,0-1 日龄水蚤的感染率最高 |
(Garbutt 等人, 2014 年)* |
南美白对虾(白虾) |
对虾杆状病毒 | 是的 | 随着年龄的增长,死亡率和感染率下降 |
(勒布朗和奥弗斯特里特, 1990 ) |
Zootermopsis angusticollis (白蚁) |
金龟子绿僵菌 | 是的 | 年轻龄虫的死亡率高于老龄虫和若虫 |
(罗森高斯和特拉尼洛, 2001 ) |
Anticarsia gemmataliss (绒豆毛毛虫) |
核多角体病毒 | 是的 | LD 50 随着年龄的增长而增加。第二龄幼虫比其他年龄段的幼虫更易受影响 40% |
(布西亚斯等人, 1980 ) |
Glossina morsitans morsitans (采采蝇) |
刚果锥虫和布氏锥虫 |
是的 | 成蝇在出苗后 0-1 天感染率最高,并随着年龄的增长而下降 |
(库比等人, 2006 )* |
Ploida interpunctella (印度餐口毛毛虫) |
颗粒病毒 | 是的 | 即使考虑到体重,死亡率在最年轻的龄期最高,并且随着年龄的增长而下降 |
(Sait 等人, 1994 年)* |
意大利蜜蜂( Apis mellifera ) |
臭虫(芽孢杆菌幼虫) |
是的 | 在敏感和抗性蜜蜂幼虫系中,幼虫死亡率随着接种年龄的增加而下降 |
(班布里克和罗森布勒, 1961 年) |
光滑双脐螺(蜗牛) |
曼氏血吸虫 | 不 | 感染率随宿主体型大小而降低,但考虑到宿主体型大小后,感染率不受宿主年龄的影响 |
(安德森等人, 1982 )* |
Danaus plexippus (帝王蝶) |
电核藻 |
不 | 一龄幼虫的感染率低于二龄幼虫。被感染的较年轻的龄期产生的孢子较少 |
|
| 植物 | ||||
| 西兰花 |
霜霉病(Hyaloperonospora parasitica) |
是的 | 各品种在子叶阶段对感染的抵抗力存在差异,但所有测试品种在成年阶段的抵抗力均有所增加 |
(科埃略等人, 2009 ) |
| 黄瓜 | 腐霉属 | 是的 | 年龄较大时接种的黄瓜种子对腐霉感染的抵抗力更强 |
(麦克卢尔和罗宾斯, 1942 ) |
| 燕麦 | Blumeria gramminis (白粉病) |
是的 | 成年植物的抗性已经很明确,并以乳头形成的形式出现。老叶子和老植物更强 |
(桑切斯-马丁等人, 2011 ) |
| 花生 | 花生柄锈菌 | 是的 | 随着叶龄的增加,侵染效率降低,潜伏期延长 |
(萨瓦里, 1987 ) |
| 土豆 | 马铃薯病毒PVY | 是的 | 幼苗感染率较高,老植物感染率降至零 |
(吉布森, 1991 ) |
| 土豆 | 致病疫霉(晚疫病) |
是的 | 3至6周龄易感性显着下降 |
(斯图尔特等人, 1983 ) |
| 响龙 | 最终腐霉 | 是的 | 苗期死亡率高,播种后20天接种可降低死亡率 |
(梅拉诺等人, 1970 ) |
| 小麦 | 小麦矮缩病毒 | 是的 | 随着年龄的增长,易感性急剧下降 |
(林布拉德和西格瓦尔德, 2004 ) |
| 冬小麦 | 鬼伞 ( Coprinus psychromorbidus )(棉花雪霉) |
是的 | 硬化期较长的老植物对感染和死亡的抵抗力更强 |
(高德和陈, 1987 ) |
疾病暴露风险的变化可能是不同年龄段耐药性进化的重要驱动因素。鉴于耐药性通常代价高昂(Biere & Antonovics, 1996 ;Cotter et al., 2004 ;Eraud et al., 2009 ;Freitak et al., 2003 ;Tian et al., 2003 ),选择应有利于耐药机制的表达与给定年龄的暴露风险相对应,并由成本调节(Ashby & Bruns, 2018 ;Bruns, 2019 )。例如,性不成熟的青少年不太可能患上性传播疾病,因此我们期望选择有利于后来对这些类型的疾病产生抵抗力。在许多生物体中,老年人占据的领地更大,消耗的食物更多,从而增加了他们摄入口腔传播病原体的机会(Elliot 等, 2002 ;Garbutt 等, 2014 )。在群居动物中,成年动物通常比幼年动物具有更高的接触率和更高的网络连接性(Carter et al., 2013 ;Rimbach et al., 2015 ),这可能会增加直接传播疾病的风险。相比之下,成年人感染沃尔巴克氏体等垂直传播疾病的风险显然较低,尽管接种研究表明成年人可以被感染(Werren等, 2008 )。
一个关键问题是,宿主是否以及在多大程度上有能力应对这些特定年龄的选择差异。抵抗力可以在成年和青少年阶段独立进化吗?迄今为止,尽管抵抗力年龄依赖性现象已有充分记录,但我们对其潜在遗传学的信息却很少。据推测,幼虫阶段的发育资源限制可能会强烈限制宿主在该阶段进化出抗性的能力(Boege & Marquis, 2005 ;McDade, 2003 )。然而,在人类(Hill 等, 1991 ;Mockenhaupt 等, 2006 )和其他动物(Cotter 等, 2004 ;Gauly 等, 2002 )中,幼年阶段疾病的遗传变异已得到充分记录。和植物(Chung 等, 2012 ;Jarosz 和 Burdon, 1990 ),表明宿主群体有能力在年轻时进化出抗性。然而,关于青少年和成人抵抗力的相关程度,我们的信息要少得多。在果蝇中,Lesser 等人。 ( 2006 ) 发现个体自交系在羽化后 1 周龄和 4 周龄时清除大肠杆菌感染的能力没有相关性,表明这两个年龄段的抗病性背后有不同的位点。在小麦和其他粮食作物中,全基因组关联研究表明,在幼苗和成年阶段对锈菌真菌具有抗性的位点存在不同程度的重叠(Gao 等人, 2016 年;Liu 等人, 2017 年;Panter 和 Jones,泽格耶等人。, 2014 )。然而,作物的抗性遗传学受到选择性育种的强烈影响,可能无法准确反映自然界的进化过程。
在这里,我们研究了三种野生植物( Silene latifolia 、 Silene vulgaris和Dianthus pavonius )的幼苗和成虫对自然发生的真菌病害(由Microbotryum真菌引起的花药黑粉病)的遗传相关性。在这些宿主中,小葡萄球菌的感染导致花朵从花药中产生孢子,代替花粉,并且子房败育,导致植物完全不育(Alexander & Maltby, 1990 )。我们在之前对孔雀石斛的研究中表明,成年植物比幼苗对接种感染的抵抗力要强得多(Bruns et al., 2017 )。此外,向幼苗的传播是疾病动态的主要驱动因素,我们的高患病人群流行病学模型表明,80%的传播事件是通过幼苗感染发生的(Bruns等, 2017 )。这些结果表明该系统中年龄依赖性抗性变异具有很强的生态重要性。一个潜在的原因是,由于开花(以及带有孢子的传粉者的造访)带来的暴露风险增加,在成虫阶段对抗性的选择更强。幼虫仍然可以通过附近患病植物的孢子被动、空中沉积来接触病原体,但这种传播模式具有更陡的传播梯度(Bruns等人, 2017 )。
在本研究中,我们使用接种实验来研究D. pavonius以及其他两种常受花药黑粉病影响的寄主物种在幼年和成年阶段的科级抗性之间的相关性,目的是评估年龄的普遍性- 抵抗力的特定遗传差异。强烈的遗传相关性表明,相同或相关的基因座可能是两个年龄段抗性的基础,从而限制了对青少年和成年阶段抗病性选择的独立反应。相比之下,弱相关性和强的年龄×家庭相互作用表明,不同的位点是青少年和成年阶段抵抗力的基础,具有年龄特异性防御独立进化的潜力。在一个物种( S. latifolia )中,我们还进行了后续实验,以区分年龄本身与发育阶段(营养与生殖)对抵抗力的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号