Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2022-07-16 , DOI: 10.1016/j.jwpe.2022.102989 Monu Verma , Waseem Ahmad , Ju-Hyun Park , Vinod Kumar , Mikhail S. Vlaskin , Dipti Vaya , Hyunook Kim
|
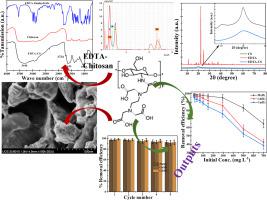
Potentially toxic heavy metals commonly exist in industrial wastewaters, presenting a critical global health threat to human health and environment, therefore, making their treatment is more challenging. In this study, an attractive polymer composite (CS-EDTA) adsorbent was developed by immobilization of ethylenediaminetetraacetic acid (EDTA) onto chitosan (CS) through cross-linking for the adsorptive removal of multiple heavy metals from industrial wastewater. The adsorption of heavy metals, i.e., Pb(II), Cd(II), and Cu(II) onto the developed composite was investigated by performing batch experiments with varying contact time and metal ion concentration in the mono-component system. The adsorption data fitted to the monolayer Langmuir isotherm; the maximum adsorption capacities were calculated as 370.37 ± 14.26, 243.90 ± 12.47, and 227.27 ± 15.33 mg g−1 for Pb(II), Cd(II), and Cu(II), respectively. The kinetics of the adsorption followed the pseudo-second order (PSO) models, and obtained the rate constant values of 0.009 ± 0.0004, 0.001 ± 0.0001, and 0.0007 ± 0.0001 g mg−1 min−1 for Pb(II), Cd(II), and Cu(II), respectively. The adsorption of the heavy metals was attributed to the electrostatic interactions between the metals and different functional groups ( OH,
OH,  NH2, and
NH2, and  COOH) of the adsorbent and complexation with EDTA, which was confirmed by the elemental mapping, EDS and FT-IR techniques. The reusability of the polymer composite was also tested and obtained >92 % efficiency even after five consecutive adsorption-desorption cycles which clearly demonstrating the high stability of the adsorbent. Lastly, the developed polymer composite was applied for real industrial wastewater. Excellent removal efficiencies (>84 %) were obtained, which makes it a potential candidate for the removal of heavy metals from real industrial wastewater.
COOH) of the adsorbent and complexation with EDTA, which was confirmed by the elemental mapping, EDS and FT-IR techniques. The reusability of the polymer composite was also tested and obtained >92 % efficiency even after five consecutive adsorption-desorption cycles which clearly demonstrating the high stability of the adsorbent. Lastly, the developed polymer composite was applied for real industrial wastewater. Excellent removal efficiencies (>84 %) were obtained, which makes it a potential candidate for the removal of heavy metals from real industrial wastewater.
中文翻译:

使用 EDTA 的壳聚糖一步功能化:多种重金属吸附的动力学和等温线建模及其机制
工业废水中普遍存在潜在有毒重金属,对人类健康和环境构成严重的全球健康威胁,因此,使其处理更具挑战性。在这项研究中,通过交联将乙二胺四乙酸(EDTA)固定在壳聚糖(CS)上,开发了一种有吸引力的聚合物复合材料(CS-EDTA)吸附剂,用于吸附去除工业废水中的多种重金属。通过在单组分系统中进行不同接触时间和金属离子浓度的批量实验,研究了重金属,即 Pb(II)、Cd(II) 和 Cu(II) 在开发的复合材料上的吸附。符合单层朗缪尔等温线的吸附数据;最大吸附容量计算为 370.37 ± 14.26、243.90 ± 12.47 和 227.27 ± 15。Pb(II)、Cd(II) 和 Cu(II) 分别为-1 。吸附动力学遵循准二级(PSO) 模型,对于Pb (II)、Cd( II) 和 Cu(II)。重金属的吸附归因于金属与不同官能团( OH、
OH、 NH 2和
NH 2和 COOH) 的吸附剂和与 EDTA 的络合,这通过元素映射、EDS 和 FT-IR 技术得到证实。聚合物复合材料的可重复使用性也进行了测试,即使经过五个连续的吸附-解吸循环后仍能获得 >92% 的效率,这清楚地表明了吸附剂的高稳定性。最后,将开发的聚合物复合材料应用于实际工业废水。获得了出色的去除效率 (>84 %),这使其成为从实际工业废水中去除重金属的潜在候选者。
COOH) 的吸附剂和与 EDTA 的络合,这通过元素映射、EDS 和 FT-IR 技术得到证实。聚合物复合材料的可重复使用性也进行了测试,即使经过五个连续的吸附-解吸循环后仍能获得 >92% 的效率,这清楚地表明了吸附剂的高稳定性。最后,将开发的聚合物复合材料应用于实际工业废水。获得了出色的去除效率 (>84 %),这使其成为从实际工业废水中去除重金属的潜在候选者。










































 京公网安备 11010802027423号
京公网安备 11010802027423号