当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an Optimized Synthetic Process for an Antiobesity Drug Candidate (S-234462) Featuring Mild Chlorination of Benzoxazolone and In Situ IR Monitoring of a Mitsunobu Reaction
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-07-14 , DOI: 10.1021/acs.oprd.2c00150 Shinichi Oda 1 , Yuki Fukui 1 , Yasuyuki Hozumi 1 , Yoshiyuki Takeuchi 1 , Masahiro Hosoya 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-07-14 , DOI: 10.1021/acs.oprd.2c00150 Shinichi Oda 1 , Yuki Fukui 1 , Yasuyuki Hozumi 1 , Yoshiyuki Takeuchi 1 , Masahiro Hosoya 1
Affiliation
|
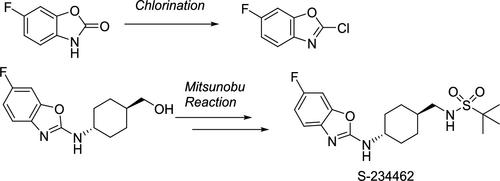
Here, we outline the manufacturing process history of S-234462. For the optimized process, 6-fluorobenzoxazolone, ((1r,4r)-4-aminocyclohexyl)methanol, and ethyl(tert-butylsulfonyl)carbamate were selected as starting materials. The first key to successful process development was the discovery of mild chlorination conditions of 6-fluorobenzoxazolone using phosphorus pentachloride and polyphosphoric acid. The second key was the establishment of N-alkylation by the Mitsunobu reaction, which did not require excess ethyl(tert-butylsulfonyl)carbamate. In addition, kinetic study of the Mitsunobu reaction using in situ IR analysis enabled control of the reaction conversion.
中文翻译:

抗肥胖候选药物 (S-234462) 的优化合成工艺开发,具有苯并恶唑酮的轻度氯化和 Mitsunobu 反应的原位 IR 监测
在这里,我们概述了 S-234462 的制造过程历史。优化工艺以6-氟苯并恶唑酮、((1r,4r)-4-氨基环己基)甲醇和(叔丁基磺酰基)氨基甲酸乙酯为起始原料。成功工艺开发的第一个关键是发现了使用五氯化磷和多磷酸的 6-氟苯并恶唑酮的温和氯化条件。第二个关键是通过Mitsunobu反应建立N-烷基化,不需要过量的(叔丁基磺酰基)氨基甲酸乙酯。此外,使用原位 IR 分析对 Mitsunobu 反应的动力学研究能够控制反应转化率。
更新日期:2022-07-14
中文翻译:

抗肥胖候选药物 (S-234462) 的优化合成工艺开发,具有苯并恶唑酮的轻度氯化和 Mitsunobu 反应的原位 IR 监测
在这里,我们概述了 S-234462 的制造过程历史。优化工艺以6-氟苯并恶唑酮、((1r,4r)-4-氨基环己基)甲醇和(叔丁基磺酰基)氨基甲酸乙酯为起始原料。成功工艺开发的第一个关键是发现了使用五氯化磷和多磷酸的 6-氟苯并恶唑酮的温和氯化条件。第二个关键是通过Mitsunobu反应建立N-烷基化,不需要过量的(叔丁基磺酰基)氨基甲酸乙酯。此外,使用原位 IR 分析对 Mitsunobu 反应的动力学研究能够控制反应转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号