Minerals Engineering ( IF 4.9 ) Pub Date : 2022-07-13 , DOI: 10.1016/j.mineng.2022.107721 Reshad Hesami , Ali Ahmadi , Mohammad Raouf Hosseini , Zahra Manafi
|
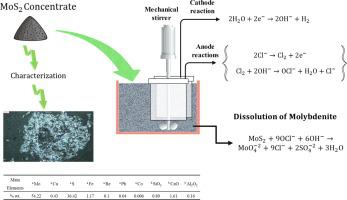
The kinetics of molybdenum and copper extraction from Sarcheshmeh molybdenite concentrate (Kerman, Iran) was studied by electroleaching process in batch mode. The effects of several critical parameters including sodium chloride concentration, pH, temperature, time, operating voltage, and the sodium carbonate addition on the molybdenum and copper extraction from the concentrate were investigated. Results showed that the molybdenum extraction significantly increased by increasing sodium chloride concentration, temperature, time, operating voltage, and sodium carbonate addition during the process. While with increasing pH, the molybdenum extraction decreased. Maximum molybdenum extraction (89%) was obtained at the sodium hypochlorite concentration of 30 g/L, the pH of 9, the temperature of 50 °C, operating voltage of 5 V, and reaction time of 4 h. Copper extraction was determined 17.5% in 30 min, which decreased to 3.9% over 8 h. It was found that carbonate ions added to the electroleaching process had a significant effect on molybdenum extraction. Also, carbonate ions might prevent hypochlorite decomposition by stabilizing copper (III) ions. The catalytic compounds of copper precipitate simultaneously with the catalytic decomposition of the hypochlorite. Kinetic studies have shown that the dissolution of molybdenite concentrate is described by a shrinking core model controlled by a chemical reaction. The activation energy for the electroleaching process of molybdenite concentrate was determined to be 29.4 kJ/mol. It seems that electroleaching is an efficient, green, and selective process for treating molybdenite concentrates.
中文翻译:

Sarcheshmeh铜配合物辉钼精矿在氯化物介质中的电浸动力学
从 Sarcheshmeh 辉钼矿中提取钼和铜的动力学精矿(Kerman,伊朗)采用间歇式电解浸出工艺进行了研究。研究了几个关键参数,包括氯化钠浓度、pH、温度、时间、操作电压和碳酸钠添加量对从精矿中提取钼和铜的影响。结果表明,在此过程中,随着氯化钠浓度、温度、时间、工作电压和碳酸钠添加量的增加,钼的提取率显着提高。而随着 pH 值的增加,钼的提取量下降。在次氯酸钠浓度为 30 g/L、pH 为 9、温度为 50 °C、工作电压为 5 V、反应时间为 4 h 时,钼提取率最高(89%)。在 30 分钟内测定铜提取率为 17.5%,降至 3。9% 超过 8 小时。发现在电浸过程中加入碳酸根离子对钼的提取有显着影响。此外,碳酸根离子可能会通过稳定铜 (III) 离子来防止次氯酸盐分解。铜的催化化合物与次氯酸盐的催化分解同时沉淀。动力学研究表明,辉钼矿精矿的溶解是通过由化学反应控制的收缩核心模型来描述的。辉钼精矿电浸过程的活化能确定为 29.4 kJ/mol。看来电浸出是一种处理辉钼矿精矿的高效、绿色和选择性工艺。碳酸根离子可能通过稳定铜 (III) 离子来防止次氯酸盐分解。铜的催化化合物与次氯酸盐的催化分解同时沉淀。动力学研究表明,辉钼矿精矿的溶解是通过由化学反应控制的收缩核心模型来描述的。辉钼精矿电浸过程的活化能确定为 29.4 kJ/mol。看来电浸出是一种处理辉钼矿精矿的高效、绿色和选择性工艺。碳酸根离子可能通过稳定铜 (III) 离子来防止次氯酸盐分解。铜的催化化合物与次氯酸盐的催化分解同时沉淀。动力学研究表明,辉钼矿精矿的溶解是通过由化学反应控制的收缩核心模型来描述的。辉钼精矿电浸过程的活化能确定为 29.4 kJ/mol。看来电浸出是一种处理辉钼矿精矿的高效、绿色和选择性工艺。动力学研究表明,辉钼矿精矿的溶解是通过由化学反应控制的收缩核心模型来描述的。辉钼精矿电浸过程的活化能确定为 29.4 kJ/mol。看来电浸出是一种处理辉钼矿精矿的高效、绿色和选择性工艺。动力学研究表明,辉钼矿精矿的溶解是通过由化学反应控制的收缩核心模型来描述的。辉钼精矿电浸过程的活化能确定为 29.4 kJ/mol。看来电浸出是一种处理辉钼矿精矿的高效、绿色和选择性工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号