Journal of Controlled Release ( IF 10.5 ) Pub Date : 2022-07-12 , DOI: 10.1016/j.jconrel.2022.07.004 Heejin Jun , Eunjung Jang , Hansol Kim , Mirae Yeo , Seong Guk Park , Jaehyeok Lee , Kyeong Jin Shin , Young Chan Chae , Sebyung Kang , Eunhee Kim
|
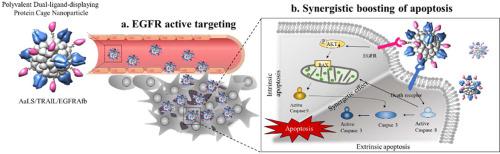
The TNF-related apoptosis-inducing ligand (TRAIL) is a promising anticancer drug candidate because it selectively binds to the proapoptotic death receptors, which are frequently overexpressed in a wide range of cancer cells, subsequently inducing strong apoptosis in these cells. However, the therapeutic benefit of TRAIL has not been clearly proven, mainly because of its poor pharmacokinetic characteristics and frequent resistance to its application caused by the activation of a survival signal via the EGF/epidermal growth factor receptor (EGFR) signaling pathway. Here, a lumazine synthase protein cage nanoparticle isolated from Aquifex aeolicus (AaLS) was used as a multiple ligand-displaying nanoplatform to display polyvalently both TRAIL and EGFR binding affibody molecules (EGFRAfb) via a SpyTag/SpyCatcher protein-ligation system, to form AaLS/TRAIL/EGFRAfb. The dual-ligand-displaying AaLS/TRAIL/EGFRAfb exhibited a dramatically enhanced cytotoxicity on TRAIL-resistant and EGFR-overexpressing A431 cancer cells in vitro, effectively disrupting the EGF-mediated EGFR survival signaling pathway by blocking EGF/EGFR binding as well as strongly activating both the extrinsic and intrinsic apoptotic pathways synergistically. The AaLS/TRAIL/EGFRAfb selectively targeted A431 cancer cells in vitro and actively reached the tumor sites in vivo. The A431 tumor-bearing mice treated with AaLS/TRAIL/EGFRAfb exhibited a significant suppression of the tumor growth without any significant side effects. Collectively, these findings showed that the AaLS/TRAIL/EGFRAfb could be used as an effective protein-based therapeutic for treating EGFR-positive cancers, which are difficult to manage using mono-therapeutic approaches.
中文翻译:

蛋白质纳米颗粒上的 TRAIL 和 EGFR 亲和体双重展示协同抑制肿瘤生长
TNF 相关的凋亡诱导配体 (TRAIL) 是一种很有前途的抗癌候选药物,因为它选择性地结合促凋亡死亡受体,这些受体经常在多种癌细胞中过表达,随后在这些细胞中诱导强烈的凋亡。然而,TRAIL 的治疗益处尚未得到明确证明,主要是因为其药代动力学特性较差,并且由于通过EGF/表皮生长因子受体 (EGFR) 信号通路激活存活信号而导致对其应用的频繁耐药。在这里,从Aquifex aeolicus (AaLS) 中分离出的 lumazine 合酶蛋白笼纳米颗粒用作多配体展示纳米平台,以多价展示 TRAIL 和 EGFR 结合亲和体分子 (EGFRAfb)通过SpyTag/SpyCatcher 蛋白连接系统,形成 AaLS/TRAIL/EGFRAfb。展示双配体的 AaLS/TRAIL/EGFRAfb在体外对 TRAIL 抗性和过度表达 EGFR 的 A431 癌细胞表现出显着增强的细胞毒性,通过阻断 EGF/EGFR 结合有效地破坏 EGF 介导的 EGFR 存活信号通路以及强烈协同激活外在和内在的凋亡途径。AaLS/TRAIL/EGFRAfb在体外选择性靶向 A431 癌细胞并在体内主动到达肿瘤部位. 用 AaLS/TRAIL/EGFRAfb 治疗的 A431 荷瘤小鼠表现出对肿瘤生长的显着抑制,而没有任何显着的副作用。总的来说,这些研究结果表明,AaLS/TRAIL/EGFRAfb 可用作治疗 EGFR 阳性癌症的有效蛋白质治疗剂,这种癌症难以使用单一治疗方法进行管理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号