Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2022-07-11 , DOI: 10.1016/j.jcou.2022.102128 Yuxin Liu , Liwen Li , Ruoyu Zhang , Yonghua Guo , Hua Wang , Qingfeng Ge , Xinli Zhu
|
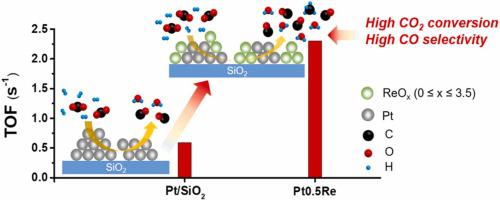
Catalytic reduction of CO2 with renewable H2 to CO via the reverse water gas shift (RWGS) reaction is an attractive approach to recycle CO2 and control net emission of CO2 to atmosphere. However, low activity and high CH4 selectivity at low temperatures limited the practical implementation of the reaction. Herein, Pt-Re/SiO2 catalysts with varying amount of Re were prepared with co-impregnation and tested for the RWGS reaction. Characterization results indicated that the oxophilic ReOx (0 ≤ x ≤ 3.5) located in close proximity to Pt particle, modified the surface of Pt by both partial coverage and electronic interaction, resulting in the reduced number of sites for and weakened strength of CO adsorption. At 400 °C, the turnover frequency (2.30 s−1) on the optimal Pt-Re/SiO2 catalyst (Pt/Re = 1.91) is 3.9 times higher than that on Pt/SiO2 under differential conditions, and the apparent activation energy is lowered. In contrast to Re/SiO2 which produces significant amount of CH4, the CO selectivity on Pt-Re/SiO2 maintained > 96.2 % under integral conditions. Reaction order analysis revealed that Pt facilitates H2 activation whereas the oxophilic ReOx enhances CO2 adsorption and activation. The perimeter sites at the Pt/ReOx interface with a balanced hydrogenation and C-O cleavage properties synergistically improve the RWGS activity while inhibiting CH4 production.
中文翻译:

协同增强 Pt-Re/SiO2 催化剂逆水煤气变换反应的活性和选择性
通过反向水煤气变换(RWGS)反应用可再生的H 2催化还原CO 2为CO 是回收CO 2和控制CO 2向大气的净排放的有吸引力的方法。然而,低温下的低活性和高 CH 4选择性限制了该反应的实际实施。在此,通过共浸渍制备了具有不同 Re 量的Pt-Re/SiO 2催化剂,并测试了 RWGS 反应。表征结果表明,亲氧 ReO x(0≤x≤3.5)位于Pt颗粒附近,通过部分覆盖和电子相互作用对Pt表面进行修饰,导致CO吸附位点数量减少,强度减弱。在400 ℃下,最佳Pt-Re/SiO 2催化剂(Pt/Re = 1.91)的转换频率(2.30 s -1 )是Pt/SiO 2在不同条件下的3.9倍,表观活化能量降低。与产生大量 CH 4的 Re/SiO 2相比,Pt-Re/SiO 2上的 CO 选择性在积分条件下保持 > 96.2 %。反应顺序分析表明 Pt 促进 H 2而亲氧的ReO x增强了CO 2的吸附和活化。Pt/ReO x界面的周边位点具有平衡的氢化和 CO 裂解特性,可协同提高 RWGS 活性,同时抑制 CH 4的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号