Molecular Therapy ( IF 12.1 ) Pub Date : 2022-07-12 , DOI: 10.1016/j.ymthe.2022.07.007 Sanne Bevers 1 , Sander A A Kooijmans 2 , Elien Van de Velde 3 , Martijn J W Evers 2 , Sofie Seghers 3 , Jerney J J M Gitz-Francois 2 , Nicky C H van Kronenburg 4 , Marcel H A M Fens 4 , Enrico Mastrobattista 4 , Lucie Hassler 5 , Helena Sork 6 , Taavi Lehto 7 , Kariem E Ahmed 8 , Samir El Andaloussi 8 , Katja Fiedler 3 , Karine Breckpot 9 , Michael Maes 3 , Diane Van Hoorick 3 , Thierry Bastogne 10 , Raymond M Schiffelers 2 , Stefaan De Koker 3
|
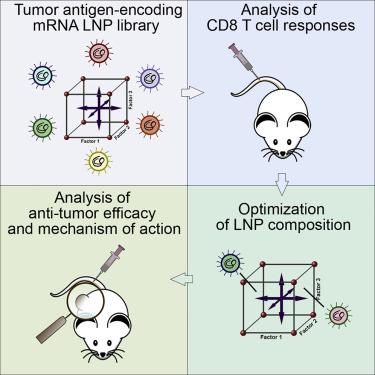
mRNA vaccines have recently proved to be highly effective against SARS-CoV-2. Key to their success is the lipid-based nanoparticle (LNP), which enables efficient mRNA expression and endows the vaccine with adjuvant properties that drive potent antibody responses. Effective cancer vaccines require long-lived, qualitative CD8 T cell responses instead of antibody responses. Systemic vaccination appears to be the most effective route, but necessitates adaptation of LNP composition to deliver mRNA to antigen-presenting cells. Using a design-of-experiments methodology, we tailored mRNA-LNP compositions to achieve high-magnitude tumor-specific CD8 T cell responses within a single round of optimization. Optimized LNP compositions resulted in enhanced mRNA uptake by multiple splenic immune cell populations. Type I interferon and phagocytes were found to be essential for the T cell response. Surprisingly, we also discovered a yet unidentified role of B cells in stimulating the vaccine-elicited CD8 T cell response. Optimized LNPs displayed a similar, spleen-centered biodistribution profile in non-human primates and did not trigger histopathological changes in liver and spleen, warranting their further assessment in clinical studies. Taken together, our study clarifies the relationship between nanoparticle composition and their T cell stimulatory capacity and provides novel insights into the underlying mechanisms of effective mRNA-LNP-based antitumor immunotherapy.
中文翻译:

针对全身免疫调整的 mRNA-LNP 疫苗通过接合脾免疫细胞诱导强大的抗肿瘤免疫
最近证明 mRNA 疫苗对 SARS-CoV-2 非常有效。他们成功的关键是基于脂质的纳米颗粒 (LNP),它能够实现有效的 mRNA 表达,并赋予疫苗佐剂特性,从而驱动有效的抗体反应。有效的癌症疫苗需要长寿命的、定性的 CD8 T 细胞反应而不是抗体反应。全身疫苗接种似乎是最有效的途径,但需要调整 LNP 成分以将 mRNA 递送至抗原呈递细胞。使用实验设计方法,我们定制了 mRNA-LNP 组合物,以在一轮优化中实现高幅度的肿瘤特异性 CD8 T 细胞反应。优化的 LNP 组成导致多个脾免疫细胞群对 mRNA 的摄取增强。发现 I 型干扰素和吞噬细胞对 T 细胞反应至关重要。令人惊讶的是,我们还发现了 B 细胞在刺激疫苗引发的 CD8 T 细胞反应中的一个尚未确定的作用。优化的 LNP 在非人类灵长类动物中表现出类似的以脾脏为中心的生物分布特征,并且不会引发肝脏和脾脏的组织病理学变化,值得在临床研究中进行进一步评估。总之,我们的研究阐明了纳米颗粒组成与其 T 细胞刺激能力之间的关系,并为基于 mRNA-LNP 的有效抗肿瘤免疫治疗的潜在机制提供了新的见解。优化的 LNP 在非人类灵长类动物中表现出类似的以脾脏为中心的生物分布特征,并且不会引发肝脏和脾脏的组织病理学变化,值得在临床研究中进行进一步评估。总之,我们的研究阐明了纳米颗粒组成与其 T 细胞刺激能力之间的关系,并为基于 mRNA-LNP 的有效抗肿瘤免疫治疗的潜在机制提供了新的见解。优化的 LNP 在非人类灵长类动物中表现出类似的以脾脏为中心的生物分布特征,并且不会引发肝脏和脾脏的组织病理学变化,值得在临床研究中进行进一步评估。总之,我们的研究阐明了纳米颗粒组成与其 T 细胞刺激能力之间的关系,并为基于 mRNA-LNP 的有效抗肿瘤免疫治疗的潜在机制提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号