Molecular Therapy ( IF 12.1 ) Pub Date : 2022-07-12 , DOI: 10.1016/j.ymthe.2022.07.009 Anthea Wirges 1 , Mario Bunse 2 , Jara J Joedicke 1 , Eric Blanc 3 , Venugopal Gudipati 4 , Michael W Moles 1 , Hiroshi Shiku 5 , Dieter Beule 3 , Johannes B Huppa 4 , Uta E Höpken 2 , Armin Rehm 1
|
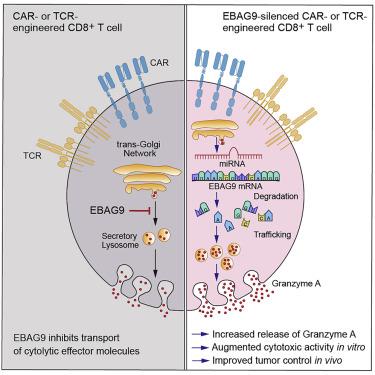
Chimeric antigen receptor (CAR) T cells have revolutionized treatment of B cell malignancies. However, enhancing the efficacy of engineered T cells without compromising their safety is warranted. The estrogen receptor-binding fragment-associated antigen 9 (EBAG9) inhibits release of cytolytic enzymes from cytotoxic T lymphocytes. Here, we examined the potency of EBAG9 silencing for the improvement of adoptive T cell therapy. MicroRNA (miRNA)-mediated EBAG9 downregulation in transplanted cytolytic CD8+ T cells (CTLs) from immunized mice improved their cytolytic competence in a tumor model. In tolerant female recipient mice that received organ transplants, a minor histocompatibility antigen was turned into a rejection antigen by Ebag9 deletion, indicating an immune checkpoint function for EBAG9. Considerably fewer EBAG9-silenced human CAR T cells were needed for tumor growth control in a xenotransplantation model. Transcriptome profiling did not reveal additional risks regarding genotoxicity or aberrant differentiation. A single-step retrovirus transduction process links CAR or TCR expression with miRNA-mediated EBAG9 downregulation. Despite higher cytolytic efficacy, release of cytokines associated with cytokine release syndrome remains unaffected. Collectively, EBAG9 silencing enhances effector capacity of TCR- and CAR-engineered T cells, results in improved tumor eradication, facilitates efficient manufacturing, and decreases the therapeutic dose.
中文翻译:

EBAG9沉默发挥免疫检查点功能而不加重不良反应
嵌合抗原受体 (CAR) T 细胞彻底改变了 B 细胞恶性肿瘤的治疗。然而,在不损害其安全性的情况下增强工程化 T 细胞的功效是有必要的。雌激素受体结合片段相关抗原 9 (EBAG9) 抑制细胞毒性 T 淋巴细胞释放溶细胞酶。在这里,我们检查了 EBAG9 沉默对于改善过继性 T 细胞疗法的效力。在肿瘤模型中,来自免疫小鼠的移植溶细胞 CD8+ T 细胞 (CTL) 中 MicroRNA (miRNA) 介导的 EBAG9 下调提高了它们的溶细胞能力。在接受器官移植的耐受雌性受体小鼠中, Ebag9缺失将一种次要组织相容性抗原转变为排斥抗原,表明 EBAG9 具有免疫检查点功能。在异种移植模型中控制肿瘤生长所需的 EBAG9 沉默的人类 CAR T 细胞要少得多。转录组分析没有揭示有关基因毒性或异常分化的额外风险。单步逆转录病毒转导过程将 CAR 或 TCR 表达与 miRNA 介导的 EBAG9 下调联系起来。尽管溶细胞功效较高,但与细胞因子释放综合征相关的细胞因子的释放仍然不受影响。总的来说,EBAG9 沉默增强了 TCR 和 CAR 工程 T 细胞的效应能力,改善肿瘤根除,促进高效生产,并降低治疗剂量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号