Acta Neuropathologica ( IF 9.3 ) Pub Date : 2022-07-12 , DOI: 10.1007/s00401-022-02461-0 Grace I Hallinan 1 , Kadir A Ozcan 2 , Md Rejaul Hoq 2 , Laura Cracco 1 , Frank S Vago 2 , Sakshibeedu R Bharath 2 , Daoyi Li 2 , Max Jacobsen 1 , Emma H Doud 3 , Amber L Mosley 3, 4 , Anllely Fernandez 1 , Holly J Garringer 1 , Wen Jiang 2 , Bernardino Ghetti 1 , Ruben Vidal 1, 5
|
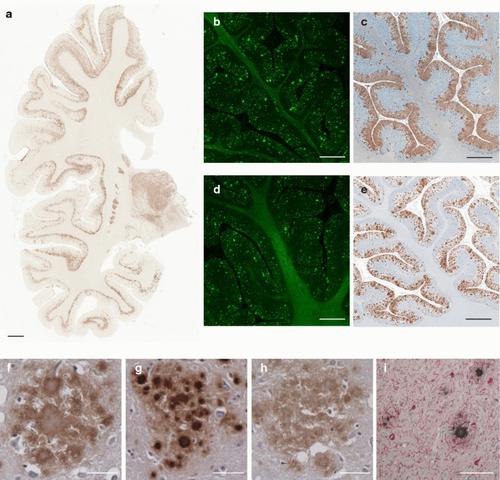
Prion protein (PrP) aggregation and formation of PrP amyloid (APrP) are central events in the pathogenesis of prion diseases. In the dominantly inherited prion protein amyloidosis known as Gerstmann–Sträussler–Scheinker (GSS) disease, plaques made of PrP amyloid are present throughout the brain. The c.593t > c mutation in the prion protein gene (PRNP) results in a phenylalanine to serine amino acid substitution at PrP residue 198 (F198S) and causes the most severe amyloidosis among GSS variants. It has been shown that neurodegeneration in this disease is associated with the presence of extracellular APrP plaques and neuronal intracytoplasmic Tau inclusions, that have been shown to contain paired helical filaments identical to those found in Alzheimer disease. Using cryogenic electron microscopy (cryo-EM), we determined for the first time the structures of filaments of human APrP, isolated post-mortem from the brain of two symptomatic PRNP F198S mutation carriers. We report that in GSS (F198S) APrP filaments are composed of dimeric, trimeric and tetrameric left-handed protofilaments with their protomers sharing a common protein fold. The protomers in the cross-β spines consist of 62 amino acids and span from glycine 80 to phenylalanine 141, adopting a previously unseen spiral fold with a thicker outer layer and a thinner inner layer. Each protomer comprises nine short β-strands, with the β1 and β8 strands, as well as the β4 and β9 strands, forming a steric zipper. The data obtained by cryo-EM provide insights into the structural complexity of the PrP filament in a dominantly inherited human PrP amyloidosis. The novel findings highlight the urgency of extending our knowledge of the filaments' structures that may underlie distinct clinical and pathologic phenotypes of human neurodegenerative diseases.
中文翻译:

Gerstmann-Sträussler-Scheinker 病朊病毒蛋白丝的冷冻电镜结构
朊病毒蛋白 (PrP) 聚集和 PrP 淀粉样蛋白 (APrP) 的形成是朊病毒疾病发病机制的核心事件。在被称为 Gerstmann-Sträussler-Scheinker (GSS) 疾病的显性遗传性朊病毒蛋白淀粉样变性中,由 PrP 淀粉样蛋白构成的斑块存在于整个大脑中。朊病毒蛋白基因 ( PRNP )中的 c.593t > c 突变导致 PrP 残基 198 (F198S) 处苯丙氨酸被丝氨酸取代,并导致 GSS 变体中最严重的淀粉样变性。研究表明,这种疾病中的神经变性与细胞外 APrP 斑块和神经元胞质内 Tau 包涵体的存在有关,这些包涵体已被证明含有与阿尔茨海默病中发现的相同的成对螺旋丝。使用低温电子显微镜 (cryo-EM),我们首次确定了人 APrP 细丝的结构,该细丝是从两名有症状的 PRNP F198S 突变携带者死后大脑中分离出来的。我们报道,在 GSS (F198S) 中,APrP 丝由二聚体、三聚体和四聚体左手原丝组成,其原聚体共享共同的蛋白质折叠。交叉β棘中的原体由62个氨基酸组成,从甘氨酸80到苯丙氨酸141,采用以前未见过的螺旋折叠,具有较厚的外层和较薄的内层。每个原聚体包含九个短β链,其中β1和β8链以及β4和β9链形成空间拉链。冷冻电镜获得的数据提供了对显性遗传性人类 PrP 淀粉样变性中 PrP 丝结构复杂性的深入了解。这些新发现强调了扩展我们对丝结构的认识的紧迫性,丝结构可能是人类神经退行性疾病不同临床和病理表型的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号