当前位置:
X-MOL 学术
›
Ann. N. Y. Acad. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational study of ion permeation through claudin-4 paracellular channels
Annals of the New York Academy of Sciences ( IF 5.2 ) Pub Date : 2022-07-10 , DOI: 10.1111/nyas.14856 Alessandro Berselli 1, 2 , Giulio Alberini 1, 3 , Fabio Benfenati 1, 3 , Luca Maragliano 1, 4
Annals of the New York Academy of Sciences ( IF 5.2 ) Pub Date : 2022-07-10 , DOI: 10.1111/nyas.14856 Alessandro Berselli 1, 2 , Giulio Alberini 1, 3 , Fabio Benfenati 1, 3 , Luca Maragliano 1, 4
Affiliation
|
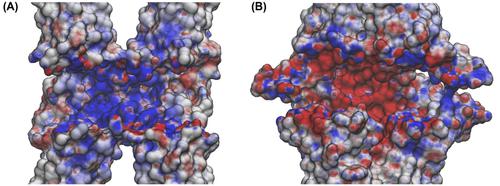
Claudins (Cldns) form a large family of protein homologs that are essential for the assembly of paracellular tight junctions (TJs), where they form channels or barriers with tissue-specific selectivity for permeants. In contrast to several family members whose physiological role has been identified, the function of claudin 4 (Cldn4) remains elusive, despite experimental evidence suggesting that it can form anion-selective TJ channels in the renal epithelium. Computational approaches have recently been employed to elucidate the molecular basis of Cldns’ function, and hence could help in clarifying the role of Cldn4. In this work, we use structural modeling and all-atom molecular dynamics simulations to transfer two previously introduced structural models of Cldn-based paracellular complexes to Cldn4 to reproduce a paracellular anion channel. Free energy calculations for ionic transport through the pores allow us to establish the thermodynamic properties driving the ion-selectivity of the structures. While one model shows a cavity permeable to chloride and repulsive to cations, the other forms barrier to the passage of all the major physiological ions. Furthermore, our results confirm the charge selectivity role of the residue Lys65 in the first extracellular loop of the protein, rationalizing Cldn4 control of paracellular permeability.
中文翻译:

claudin-4 细胞旁通道离子渗透的计算研究
Claudins (Cldns) 形成一大类蛋白质同源物,这些同源物对于细胞旁紧密连接 (TJ) 的组装至关重要,它们在此处形成通道或屏障,对渗透物具有组织特异性选择性。与已确定生理作用的几个家族成员相比,密蛋白 4 (Cldn4) 的功能仍然难以捉摸,尽管实验证据表明它可以在肾上皮细胞中形成阴离子选择性 TJ 通道。最近已采用计算方法来阐明 Cldns 功能的分子基础,因此有助于阐明 Cldn4 的作用。在这项工作中,我们使用结构建模和全原子分子动力学模拟将两个先前引入的基于 Cldn 的细胞旁复合物结构模型转移到 Cldn4 以再现细胞旁阴离子通道。通过孔隙的离子传输的自由能计算使我们能够建立驱动结构离子选择性的热力学性质。虽然一个模型显示了一个可渗透氯离子并排斥阳离子的空腔,但另一个模型形成了所有主要生理离子通过的屏障。此外,我们的结果证实了残基 Lys65 在蛋白质的第一个细胞外环中的电荷选择性作用,使 Cldn4 对细胞旁通透性的控制合理化。
更新日期:2022-07-10
中文翻译:

claudin-4 细胞旁通道离子渗透的计算研究
Claudins (Cldns) 形成一大类蛋白质同源物,这些同源物对于细胞旁紧密连接 (TJ) 的组装至关重要,它们在此处形成通道或屏障,对渗透物具有组织特异性选择性。与已确定生理作用的几个家族成员相比,密蛋白 4 (Cldn4) 的功能仍然难以捉摸,尽管实验证据表明它可以在肾上皮细胞中形成阴离子选择性 TJ 通道。最近已采用计算方法来阐明 Cldns 功能的分子基础,因此有助于阐明 Cldn4 的作用。在这项工作中,我们使用结构建模和全原子分子动力学模拟将两个先前引入的基于 Cldn 的细胞旁复合物结构模型转移到 Cldn4 以再现细胞旁阴离子通道。通过孔隙的离子传输的自由能计算使我们能够建立驱动结构离子选择性的热力学性质。虽然一个模型显示了一个可渗透氯离子并排斥阳离子的空腔,但另一个模型形成了所有主要生理离子通过的屏障。此外,我们的结果证实了残基 Lys65 在蛋白质的第一个细胞外环中的电荷选择性作用,使 Cldn4 对细胞旁通透性的控制合理化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号