当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing Artificial Metalloenzymes by Tuning of the Environment beyond the Primary Coordination Sphere
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.chemrev.2c00106 Casey Van Stappen 1 , Yunling Deng 1 , Yiwei Liu 2 , Hirbod Heidari 1 , Jing-Xiang Wang 1 , Yu Zhou 1 , Aaron P Ledray 1 , Yi Lu 1, 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.chemrev.2c00106 Casey Van Stappen 1 , Yunling Deng 1 , Yiwei Liu 2 , Hirbod Heidari 1 , Jing-Xiang Wang 1 , Yu Zhou 1 , Aaron P Ledray 1 , Yi Lu 1, 2
Affiliation
|
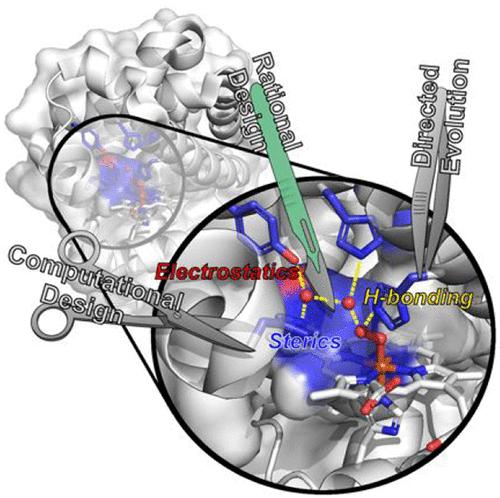
Metalloenzymes catalyze a variety of reactions using a limited number of natural amino acids and metallocofactors. Therefore, the environment beyond the primary coordination sphere must play an important role in both conferring and tuning their phenomenal catalytic properties, enabling active sites with otherwise similar primary coordination environments to perform a diverse array of biological functions. However, since the interactions beyond the primary coordination sphere are numerous and weak, it has been difficult to pinpoint structural features responsible for the tuning of activities of native enzymes. Designing artificial metalloenzymes (ArMs) offers an excellent basis to elucidate the roles of these interactions and to further develop practical biological catalysts. In this review, we highlight how the secondary coordination spheres of ArMs influence metal binding and catalysis, with particular focus on the use of native protein scaffolds as templates for the design of ArMs by either rational design aided by computational modeling, directed evolution, or a combination of both approaches. In describing successes in designing heme, nonheme Fe, and Cu metalloenzymes, heteronuclear metalloenzymes containing heme, and those ArMs containing other metal centers (including those with non-native metal ions and metallocofactors), we have summarized insights gained on how careful controls of the interactions in the secondary coordination sphere, including hydrophobic and hydrogen bonding interactions, allow the generation and tuning of these respective systems to approach, rival, and, in a few cases, exceed those of native enzymes. We have also provided an outlook on the remaining challenges in the field and future directions that will allow for a deeper understanding of the secondary coordination sphere a deeper understanding of the secondary coordintion sphere to be gained, and in turn to guide the design of a broader and more efficient variety of ArMs.
中文翻译:

通过调整主要配位球以外的环境来设计人工金属酶
金属酶使用有限数量的天然氨基酸和金属辅因子催化各种反应。因此,主要配位范围之外的环境必须在赋予和调整其非凡的催化特性方面发挥重要作用,使具有其他类似主要配位环境的活性位点能够执行多种生物功能。然而,由于主要配位范围之外的相互作用众多且微弱,因此很难确定负责调节天然酶活性的结构特征。设计人工金属酶 (ArMs) 为阐明这些相互作用的作用和进一步开发实用的生物催化剂提供了极好的基础。在这篇评论中,我们强调了 ArMs 的二级配位层如何影响金属结合和催化,特别关注使用天然蛋白质支架作为 ArMs 设计的模板,通过计算建模辅助的理性设计、定向进化或两种方法的组合. 在描述成功设计血红素、非血红素 Fe 和 Cu 金属酶、含有血红素的异核金属酶以及含有其他金属中心(包括具有非天然金属离子和金属辅因子的那些)的 ArMs 时,我们总结了关于如何仔细控制二级配位域中的相互作用,包括疏水和氢键相互作用,允许这些各自系统的产生和调整接近、竞争,并且在一些情况下超过天然酶。
更新日期:2022-07-11
中文翻译:

通过调整主要配位球以外的环境来设计人工金属酶
金属酶使用有限数量的天然氨基酸和金属辅因子催化各种反应。因此,主要配位范围之外的环境必须在赋予和调整其非凡的催化特性方面发挥重要作用,使具有其他类似主要配位环境的活性位点能够执行多种生物功能。然而,由于主要配位范围之外的相互作用众多且微弱,因此很难确定负责调节天然酶活性的结构特征。设计人工金属酶 (ArMs) 为阐明这些相互作用的作用和进一步开发实用的生物催化剂提供了极好的基础。在这篇评论中,我们强调了 ArMs 的二级配位层如何影响金属结合和催化,特别关注使用天然蛋白质支架作为 ArMs 设计的模板,通过计算建模辅助的理性设计、定向进化或两种方法的组合. 在描述成功设计血红素、非血红素 Fe 和 Cu 金属酶、含有血红素的异核金属酶以及含有其他金属中心(包括具有非天然金属离子和金属辅因子的那些)的 ArMs 时,我们总结了关于如何仔细控制二级配位域中的相互作用,包括疏水和氢键相互作用,允许这些各自系统的产生和调整接近、竞争,并且在一些情况下超过天然酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号