当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring Heterogeneous Dynamical Environment around an Ensemble of Aβ42 Peptide Monomer Conformations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.jcim.2c00593 Prabir Khatua 1 , Madhulika Gupta 2 , Sanjoy Bandyopadhyay 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.jcim.2c00593 Prabir Khatua 1 , Madhulika Gupta 2 , Sanjoy Bandyopadhyay 1
Affiliation
|
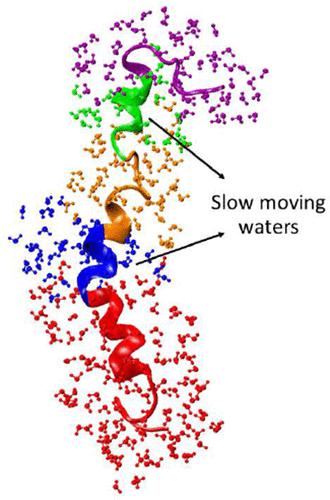
Exploring the conformational properties of amyloid β (Aβ) peptides and the role of solvent (water) in guiding the dynamical environment at their interfaces is crucial for microscopic understanding of Aβ misfolding, which is involved in causing the most common neurodegenerative disorder, i.e., Alzheimer’s disease. While numerous studies in the past have emphasized examining the conformational states of Aβ peptides, the role of water has not received much attention. Here, we have performed all-atom molecular dynamics simulations of several full-length Aβ42 peptide monomers with different initial configurations. Our efforts are directed toward probing the origin of the heterogeneous dynamics of water around various segments of the Aβ peptide, identified as the two terminal segments (N-term and C-term) and the two hydrophobic segments (hp1 and hp2), along with the central turn region interconnecting hp1 and hp2. Our results revealed that water hydrating hp1, hp2, and turn (nonterminal segments) and C-term segments exhibit nonuniformly restricted translational as well as rotational motions. The degree of such restriction has been found to be correlated with the hydrogen bond relaxation time scales at the interface. Importantly, it is revealed that the water molecules around hp1 and, to some extent, around hp2, form relatively rigid hydration layers, compared to that around the other segments. Such rigid hydration layers arise due to relatively more solid-like caging motions resulting in relatively lesser hydration entropy. As hp1 and hp2 have been demonstrated to play a central role in Aβ aggregation, we believe that distinct water dynamics in the vicinity of these two segments, as outlined in this study, can provide vital information in understanding the early stages of the onset of the aggregation process of such peptides at higher concentration that can further aid toward advances in AD therapeutics.
中文翻译:

探索 Aβ42 肽单体构象集合周围的异质动态环境
探索淀粉样蛋白 β (Aβ) 肽的构象特性以及溶剂(水)在引导其界面动态环境中的作用对于微观理解 Aβ 错误折叠至关重要,Aβ 错误折叠与导致最常见的神经退行性疾病,即阿尔茨海默氏症有关疾病。虽然过去的许多研究都强调检查 Aβ 肽的构象状态,但水的作用并没有受到太多关注。在这里,我们对几种具有不同初始构型的全长 Aβ 42肽单体进行了全原子分子动力学模拟。我们的努力旨在探索 Aβ 肽各个片段周围水的异质动力学的起源,被确定为两个末端片段 ( N -term和C - term)和两个疏水片段(hp 1 和hp 2),以及连接hp 1 和hp 2 的中心转角区域。我们的结果表明水合hp 1、hp 2 和转角(非末端片段)和C项段表现出非均匀受限的平移和旋转运动。已经发现这种限制的程度与界面处的氢键弛豫时间尺度相关。重要的是,它揭示了hp周围的水分子1 和,在某种程度上,在hp 2 附近,与其他部分相比,形成相对刚性的水化层。这种刚性水化层是由于相对更类似于固体的笼状运动而产生的,导致相对较小的水化熵。由于hp 1 和hp 2 已被证明在 Aβ 聚集中发挥核心作用,我们相信,如本研究所述,这两个部分附近不同的水动力学可以为了解发病的早期阶段提供重要信息这种肽在较高浓度下的聚集过程可以进一步有助于 AD 治疗的进步。
更新日期:2022-07-11
中文翻译:

探索 Aβ42 肽单体构象集合周围的异质动态环境
探索淀粉样蛋白 β (Aβ) 肽的构象特性以及溶剂(水)在引导其界面动态环境中的作用对于微观理解 Aβ 错误折叠至关重要,Aβ 错误折叠与导致最常见的神经退行性疾病,即阿尔茨海默氏症有关疾病。虽然过去的许多研究都强调检查 Aβ 肽的构象状态,但水的作用并没有受到太多关注。在这里,我们对几种具有不同初始构型的全长 Aβ 42肽单体进行了全原子分子动力学模拟。我们的努力旨在探索 Aβ 肽各个片段周围水的异质动力学的起源,被确定为两个末端片段 ( N -term和C - term)和两个疏水片段(hp 1 和hp 2),以及连接hp 1 和hp 2 的中心转角区域。我们的结果表明水合hp 1、hp 2 和转角(非末端片段)和C项段表现出非均匀受限的平移和旋转运动。已经发现这种限制的程度与界面处的氢键弛豫时间尺度相关。重要的是,它揭示了hp周围的水分子1 和,在某种程度上,在hp 2 附近,与其他部分相比,形成相对刚性的水化层。这种刚性水化层是由于相对更类似于固体的笼状运动而产生的,导致相对较小的水化熵。由于hp 1 和hp 2 已被证明在 Aβ 聚集中发挥核心作用,我们相信,如本研究所述,这两个部分附近不同的水动力学可以为了解发病的早期阶段提供重要信息这种肽在较高浓度下的聚集过程可以进一步有助于 AD 治疗的进步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号