Molecular Therapy ( IF 12.1 ) Pub Date : 2022-07-09 , DOI: 10.1016/j.ymthe.2022.07.003 Laura Rode 1 , Christian Bär 2 , Sonja Groß 1 , Axel Rossi 3 , Nadja Meumann 3 , Janika Viereck 1 , Naisam Abbas 1 , Ke Xiao 1 , Isabelle Riedel 1 , Anika Gietz 1 , Karina Zimmer 1 , Margarete Odenthal 4 , Hildegard Büning 5 , Thomas Thum 2
|
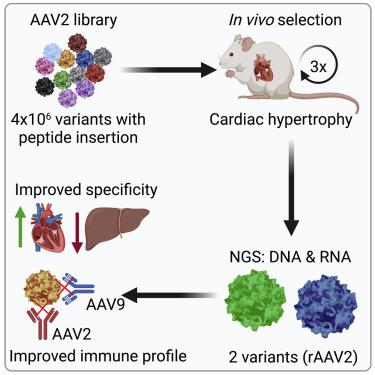
AAV vectors are promising delivery tools for human gene therapy. However, broad tissue tropism and pre-existing immunity against natural serotypes limit their clinical use. We identified two AAV capsid variants, AAV2-THGTPAD and AAV2-NLPGSGD, by in vivo AAV2 peptide display library screening in a murine model of pressure overload-induced cardiac hypertrophy. Both variants showed significantly improved efficacy in in vivo cardiomyocyte transduction compared with the parental serotype AAV2 as indicated by a higher number of AAV vector episomes in the nucleus and significant improved transduction efficiency. Both variants also outcompeted the reference serotype AAV9 regarding cardiomyocyte tropism, reaching comparable cardiac transduction efficiencies accompanied with liver de-targeting and decreased transduction efficiency of non-cardiac cells. Capsid modification influenced immunogenicity as sera of mice treated with AAV2-THGTPAD and AAV2-NLPGSGD demonstrated a poor neutralization capacity for the parental serotype and the novel variants. In a therapeutic setting, using the long non-coding RNA H19 in low vector dose conditions, novel AAV variants mediated superior anti-hypertrophic effects and revealed a further improved target-to-noise ratio, i.e., cardiomyocyte tropism. In conclusion, AAV2-THGTPAD and AAV2-NLPGSGD are promising novel tools for cardiac-directed gene therapy outperforming AAV9 regarding the specificity and therapeutic efficiency of in vivo cardiomyocyte transduction.
中文翻译:

AAV 衣壳工程鉴定出两种新变体,对心肌细胞具有改善的体内趋向性
AAV 载体是人类基因治疗的有前景的递送工具。然而,广泛的组织向性和针对自然血清型的预先存在的免疫力限制了它们的临床应用。我们通过在压力超负荷诱导的心脏肥大小鼠模型中进行体内AAV2 肽展示库筛选,鉴定了两种 AAV 衣壳变体:AAV2-THGTPAD 和 AAV2-NLPGSGD。与亲本血清型 AAV2 相比,两种变体在体内心肌细胞转导中的功效均显着提高,如细胞核中 AAV 载体附加体数量较多以及转导效率显着提高所示。两种变体在心肌细胞趋向性方面也超过了参考血清型 AAV9,达到了相当的心脏转导效率,同时伴随着肝脏脱靶和非心肌细胞转导效率的降低。衣壳修饰影响免疫原性,因为用 AAV2-THGTPAD 和 AAV2-NLPGSGD 处理的小鼠血清表现出对亲本血清型和新变体的中和能力较差。在治疗环境中,在低载体剂量条件下使用长非编码RNA H19 ,新型AAV变体介导了优异的抗肥厚作用,并揭示了进一步改善的靶噪声比,即心肌细胞向性。总之,AAV2-THGTPAD 和 AAV2-NLPGGSGD 是有前途的心脏定向基因治疗新工具,在体内心肌细胞转导的特异性和治疗效率方面优于 AAV9。











































 京公网安备 11010802027423号
京公网安备 11010802027423号