Cell Stem Cell ( IF 19.8 ) Pub Date : 2022-07-07 , DOI: 10.1016/j.stem.2022.06.001 Ruimin Xu 1 , Sen Li 2 , Qiu Wu 3 , Chong Li 3 , Manxi Jiang 4 , Lei Guo 4 , Mo Chen 3 , Lingyue Yang 3 , Xin Dong 5 , Hong Wang 3 , Chenfei Wang 5 , Xiaoyu Liu 6 , Xianghong Ou 7 , Shaorong Gao 1
|
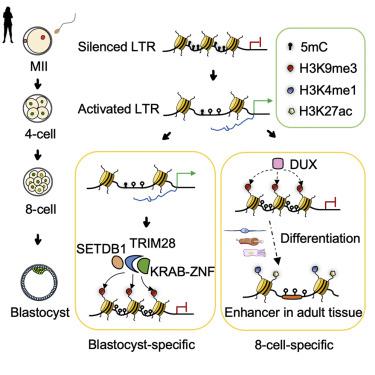
H3K9me3, as a hallmark of heterochromatin, is important for cell-fate specification. However, it remains unknown how H3K9me3 is reprogrammed during human early embryo development. Here, we profiled genome-wide H3K9me3 in human oocytes and early embryos and discovered stage-specific H3K9me3 deposition on long terminal repeats (LTRs) at the 8-cell and blastocyst stages. We found that 8-cell-specific H3K9me3 was temporarily established in enhancer-like regions, whereas blastocyst-specific H3K9me3 was more stable. DUX and multiple Krüppel-associated box domain zinc finger proteins(KRAB-ZNFs) were identified as potential factors for establishing 8C- and blastocyst-specific H3K9me3, respectively. Intriguingly, our analysis showed that stage-specific H3K9me3 allocation was attenuated by either Dux knockout or Zfp51 knockdown in mouse early embryos. Moreover, we observed the existence of H3K4me3/H3K9me3 and H3K4me3/H3K27me3 bivalent chromatin domains in human blastocysts, priming for lineage differentiation. Together, our data unveil that the epigenetic switch from DNA methylation to H3K9me3 ensures the precise regulation of retrotransposons in human pre-implantation embryos.
中文翻译:

特定阶段的 H3K9me3 占用确保人类植入前胚胎中的反转录转座子沉默
H3K9me3 作为异染色质的标志,对细胞命运的规范很重要。然而,在人类早期胚胎发育过程中如何重新编程 H3K9me3 仍然未知。在这里,我们分析了人类卵母细胞和早期胚胎中的全基因组 H3K9me3,并在 8 细胞和囊胚阶段发现了长末端重复序列 (LTR) 上的阶段特异性 H3K9me3 沉积。我们发现 8 细胞特异性 H3K9me3 暂时建立在增强子样区域,而囊胚特异性 H3K9me3 更稳定。DUX 和多个 Krüppel 相关盒域锌指蛋白 (KRAB-ZNFs) 被确定为分别建立 8C 和囊胚特异性 H3K9me3 的潜在因素。有趣的是,我们的分析表明,特定阶段的 H3K9me3 分配被Dux敲除或小鼠早期胚胎中的Zfp51敲低。此外,我们观察到人类囊胚中存在 H3K4me3/H3K9me3 和 H3K4me3/H3K27me3 二价染色质结构域,为谱系分化做好准备。总之,我们的数据揭示了从 DNA 甲基化到 H3K9me3 的表观遗传转换确保了人类植入前胚胎中反转录转座子的精确调节。










































 京公网安备 11010802027423号
京公网安备 11010802027423号