JACC: Heart Failure ( IF 10.3 ) Pub Date : 2022-07-06 , DOI: 10.1016/j.jchf.2022.04.015 Paul W Armstrong 1 , Yinggan Zheng 1 , Richard W Troughton 2 , Lars H Lund 3 , Jian Zhang 4 , Carolyn S P Lam 5 , Cynthia M Westerhout 1 , Robert O Blaustein 6 , Javed Butler 7 , Adrian F Hernandez 8 , Lothar Roessig 9 , Christopher M O'Connor 10 , Adrian A Voors 11 , Justin A Ezekowitz 1 ,
|
Background
The effect of vericiguat on sequential N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels and influence of this relationship on clinical outcomes is unknown.
Objectives
This study assessed the relationship between changes in NT-proBNP and the primary outcome (cardiovascular death or heart failure hospitalization); evaluated the effect of vericiguat on changes in NT-proBNP; and explored the association between the efficacy of vericiguat and changes in NT-proBNP.
Methods
NT-proBNP was measured at randomization and at 16, 32, 48, and 96 weeks in 4,805 of 5,050 patients. The association between NT-proBNP change at week 16 and the primary outcome was assessed. The relationship between changes in NT-proBNP and the primary outcome according to treatment group was assessed by using joint modeling and mediation analysis.
Results
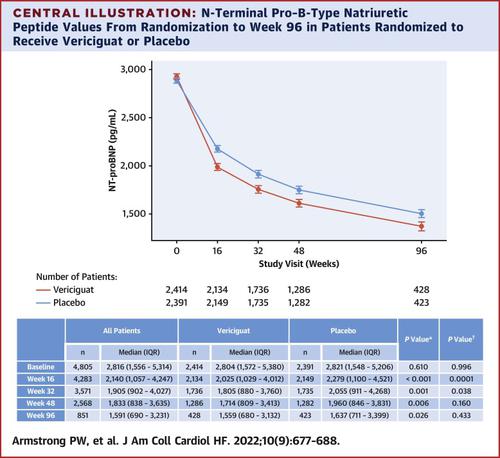
A significant and sustained decline in NT-proBNP levels was seen in both treatment groups. After week 16, NT-proBNP levels decreased more with vericiguat vs placebo (any reduction: odds ratio [OR]: 1.45 [95% CI: 1.28-1.65]; P < 0.001; ≥50% reduction: OR: 1.27 [95% CI: 1.10-1.47]; P = 0.001) and were less likely to increase (≥20% increase: OR: 0.68 [95% CI: 0.59-0.78]; P < 0.001; ≥50% increase: OR: 0.70 [95% CI: 0.59-0.82]; P < 0.001). The treatment effect related to serial NT-proBNP on the primary composite outcome was HR: 0.96 (95% CI: 0.95-0.99) at week 16, which increased to HR: 0.90 (95% CI: 0.85-0.96) at week 48; the average extent of mediation of the composite outcome related to NT-proBNP was 45%.
Conclusions
In patients with worsening HFrEF, vericiguat significantly decreased NT-proBNP levels compared with placebo. This change appeared associated with a modest relative improvement in the primary outcome of cardiovascular death or heart failure hospitalization. (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction [VICTORIA]; NCT02861534)
中文翻译:

NT-proBNP 在心力衰竭中的序贯评价
背景
Vericiguat 对连续 N 端 B 型利钠肽前体 (NT-proBNP) 水平的影响以及这种关系对临床结果的影响尚不清楚。
目标
本研究评估了 NT-proBNP 变化与主要结局(心血管死亡或心力衰竭住院)之间的关系;评估 vericiguat 对 NT-proBNP 变化的影响;并探讨了vericiguat的功效与NT-proBNP变化之间的关联。
方法
NT-proBNP 在随机分组和 16、32、48 和 96 周时对 5,050 名患者中的 4,805 名患者进行了测量。评估了第 16 周 NT-proBNP 变化与主要结果之间的关联。通过使用联合建模和中介分析评估不同治疗组的 NT-proBNP 变化与主要结果之间的关系。
结果
在两个治疗组中都观察到 NT-proBNP 水平显着和持续下降。第 16 周后,与安慰剂相比,vericiguat 组 NT-proBNP 水平降低更多(任何降低:优势比 [OR]:1.45 [95% CI:1.28-1.65];P < 0.001;≥50% 降低:OR:1.27 [95% CI:1.10-1.47];P = 0.001)并且不太可能增加(≥20% 增加:OR:0.68 [95% CI:0.59-0.78];P < 0.001;≥50% 增加:OR:0.70 [95 % CI:0.59-0.82];P < 0.001)。与系列 NT-proBNP 相关的治疗效果对主要复合结局的影响是第 16 周 HR:0.96(95% CI:0.95-0.99),第 48 周增加到 HR:0.90(95% CI:0.85-0.96);与 NT-proBNP 相关的复合结果的平均中介程度为 45%。
结论
在 HFrEF 恶化的患者中,与安慰剂相比,vericiguat 显着降低了 NT-proBNP 水平。这种变化似乎与心血管死亡或心力衰竭住院的主要结果的适度相对改善有关。(Vericiguat 对射血分数降低的心力衰竭受试者的全球研究 [VICTORIA];NCT02861534)











































 京公网安备 11010802027423号
京公网安备 11010802027423号